Abstract
Purpose
We aim to compare the efficiency and toxicity of three different 5-fluorouracil (5-FU) administration types in 5-FU, leucovorin, and oxaliplatin (FOLFOX) combination treatment for adjuvant therapy in colorectal cancer (CRC).
Methods
Five hundred and seventy patients with stage III colorectal carcinoma who received different FOLFOX regimens after curative resection were included. Patients were divided into three groups as FOLFOX-4, modified FOLFOX-6 (mFOLFOX-6), and mFOLFOX-4 for comparison of toxicity and disease-free survival (DFS) and overall survival (OS) times.
Results
Three-year DFS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 65%, 72%, and 72%, respectively. Five-year OS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 69%, 75%, and 67%, respectively. There was no statistically significant difference between the three treatment groups in terms of DFS and OS (p = 0.079, and p = 0.147, respectively). Among grade 1–2 adverse events (AE), thrombocytopenia, neuropathy, and stomatitis were more common in the mFOLFOX-6-treated group. The frequency of grade 1–2 nausea and vomiting were similar in mFOLFOX-6 (36.3% and 24%, respectively) and mFOLFOX-4 (32.4% and 24.7%, respectively) groups but were higher than that in the FOLFOX-4 (19.5% and 11.3%, respectively) group. Among the most common grade 3–4 AE, neutropenia (53.4%, 9%, and 13.5%, respectively) and diarrhea (10.5%, 2.2%, and 2.4, respectively) were more common in FOLFOX-4. The rate of anemia and febrile neutropenia was similar in treatment groups (p = 0.063, and p = 0.210, respectively).
Conclusion
In the adjuvant treatment of stage III CRC patients, three different 5-FU administration types in FOLFOX combination treatment can be used with similar efficiency and manageable toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is commonly encountered worldwide and is the third leading cause of cancer-related deaths [1]. Almost one-third of patients have locoregional lymph node metastasis at the time of diagnosis and are diagnosed at stage III [2]. When treated with surgery alone, 40-50% of patients with locoregional disease develop relapse or distant metastases due to micrometastases [3]. Adjuvant chemotherapy aims to eliminate these micrometastases and achieve cure. For the first time in the literature, Moertel et al. showed that a 12-month use of 5-fluorouracil (5-FU) and levamisole combination for stage III lymph node-positive colon cancer resulted in 33% decrease in mortality rates [4]. After the 6-month use of 5-FU and leucovorin (LV) combination was proven to have survival advantage, 5-FU/LV-based adjuvant chemotherapy for 6 months has become the standard treatment in stage III colon cancer [5]. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) study found that by adding oxaliplatin to 5-FU/LV a 7.5% increase in 5-year disease-free survival (DFS) and a 4.2% increase in 6-year overall survival (OS) was achieved, and oxaliplatin-based chemotherapy was recommended as standard postoperative treatment for these patients [6, 7].
FOLFOX-4 regimen, including the combination of oxaliplatin with LV and bolus or infusion 5-FU, is well-proven in the adjuvant therapy [7]. However, this regimen has evolved to modified FOLFOX-6 (mFOLFOX-6) and modified FOLFOX-4 (mFOLFOX-4) regimens, which are as effective with the advantage of avoiding hospital admissions by being administered through a portacath. In this study, we aim to compare these three FOLFOX regimens with the same oxaliplatin but different 5-FU administrations, which are used as adjuvant therapy in CRC, in terms of efficiency and toxicity.
Materials and methods
Study population and data collection
Five hundred and seventy patients who underwent curative surgery and received postoperative adjuvant oxaliplatin and 5-FU/LV combination therapy in 12 different oncology centers in Turkey between May 2004 and March 2019 were recruited to the study. Patients who were histopathologically diagnosed with colorectal adenocarcinoma and have stage III disease at diagnosis were included. Patients who were treated with neoadjuvant therapy for rectal cancer were excluded. The study was approved by the local Ethics Committee and conducted in accordance with the Helsinki Declaration and ethical principles (Approval no: 147/2019). Data about the sex, age at diagnosis, performance status, tumor location, stage, treatment regimen, survival, and toxicity were retrieved from patient files.
The performance status of the patients was determined according to the Eastern Cooperative Oncology Group (ECOG) performance status (PS) criteria. Staging was performed according to American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) staging Systems version 7. Toxicity was evaluated according to National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Treatment schedules
FOLFOX-4 regimen is administered every 14 days with 85 mg/m2 oxaliplatin plus 200 mg/m2 LV on day 1 and 5-FU 400 mg bolus plus 600 mg/m2 as a 22-h infusion on days 1 and 2. Modified FOLFOX-4 regimen is administered every 14 days with 85 mg/m2 oxaliplatin plus 200 mg/m2 LV and 5-FU 400 mg/m2 bolus on day 1 followed by 1600 mg/m2 5-FU as a 46-h infusion. Modified FOLFOX-6 regimen is administered every 14 days with 85 mg/m2 oxaliplatin plus 400 mg/m2 LV and 5-FU 400 mg/m2 bolus on day 1 followed by 2400 mg/m2 5-FU as a 46-h infusion. All patients with rectal primaries received adjuvant radiotherapy of 46 Gy to the pelvic area and a 4 Gy boost, for a total dose of 50 Gy, concomitantly with capecitabine 825 mg/m2 twice daily on the days of radiotherapy, or 225 mg/m2 5-FU infusion. Two cycles of chemotherapy were administered before, and the remaining 10 cycles were administered after the completion of chemoradiotherapy. Patients were followed up at 3-month intervals for the first 2 years, then at 6-month intervals until 5 years completed, with clinical visits.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 21 software (IBM Corp., Armonk, NY, USA). Descriptive analyses were presented as median, mean, and percentages. Categorical variables are given as percentages and were compared using the Chi-square or Fisher’s exact test, when appropriate. Kruskal–Wallis test was conducted to compare numerical variables among the treatment groups. A p-value of less than 0.05 was considered to show a statistically significant result. The OS and DFS rates were estimated with the Kaplan–Meier method. DFS was defined as the time from initiation of the first cycle of chemotherapy to the date of recurrence or death. OS was defined as the time from the first cycle of chemotherapy until death or last follow-up. The differences between treatment groups were assessed with the log-rank test. Parameters for which p < 0.200 in the univariate Cox models were further assessed in the multivariate Cox models. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated.
Results
Five hundred and seventy patients were evaluated in the study. Among them, 133 patients were in the FOLFOX-4 group, 267 patients were in the mFOLFOX-6 group and 170 patients were in the mFOLFOX-4 group. Three hundred and thirty-nine patients (59.5%) were male. Median age at diagnosis was 57 years (18–84) for the entire population. Median ages at diagnosis for FOLFOX-4, mFOLFOX-6 and mFOLFOX-4 groups were 56, 59, and 54.5 years, respectively (p = 0.020). There was no statistically significant difference between the groups in terms of ECOG PS, tumor location, nodal stage, and TNM stage (p = 0.109, p = 0.080, p = 0.256, and p = 0.237, respectively). On the other hand, T stage, grade, and lymphovascular invasion showed significant difference (p < 0.001, p < 0.001, and p < 0.001, respectively). In rectal cancer patients, surgical margin positivity was detected in 4 (8.9%) patients, 1 (1.3%), and 1 (1.5%) patient in FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups, respectively. The number of patients who received adjuvant radiation therapy was 63 (37%) in the mFOLFOX-4 group, 33 (25%) in the FOLFOX-4 group, and 46 (17%) in the mFOLFOX-6 group (p < 0.001). Patient characteristics are summarized in Table 1.
Survival
Median follow-up period was 62 months (4–161). Sixty-one (45.9%) patients in the FOLFOX-4 group, 76 (28.5%) patients in the mFOLFOX-6 group, and 59 (34.7%) patients in the mFOLFOX-4 group developed relapse. In patients with rectal cancer, local recurrence was detected in 5 (14.7%), 3 (3.7%), and 8 (11.7%) patients, in FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups, respectively. Fifty-seven (42.9%) patients in the FOLFOX-4 group, 59 (22.1%) patients in the mFOLFOX-6 group, and 79 (46.5%) patients in the mFOLFOX-4 group were deceased. Median value for DFS could not be reached in all patients and 3-year and 5-year DFS rates were 71% and 64.6%, respectively. Median OS of all patients was 157.5 months (95% CI, 113.8–201.2), and 3-year and 5-year OS rates were 85% and 71%, respectively.
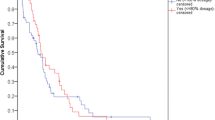
In univariate analyses, median value could not be reached for DFS according to treatment groups. Three-year DFS rates for FOLFOX-4, mFOLFOX-6 and mFOLFOX-4 groups were 65%, 72%, and 72%, respectively. No statistically significant difference was observed between the three groups in terms of DFS (p = 0.129) (Fig. 1). DFS also showed no significant difference according to age (p = 0.165), sex (p = 0.936), tumor location (p = 0.825), grade (p = 0.276), lymphovascular invasion (p = 0.383), and nodal and TNM stage (p = 0.123, and p = 0.101, respectively). ECOG PS resulted in a significant difference in DFS (p = 0.019). Patients with ECOG PS 2 had significantly lower DFS than patients with PS 0 and PS 1 (p = 0.006, and p = 0.024, respectively). T stage also resulted in a significant difference in DFS (p = 0.006). Patients with T4 disease had significantly lower DFS than patients with T2 (p = 0.013) and T3 (p = 0.007) disease. Factors associated with DFS are presented in Table 2.
When OS was evaluated according to treatment groups in univariate analyses, median value could not be reached in FOLFOX-4 and mFOLFOX-6 groups, while median OS was 139.8 months (95% CI, 122.9-156.8) in the mFOLFOX-4 group. Three-year OS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 84%, 88%, and 83%, respectively. Five-year OS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 69%, 75%, and 67%, respectively. There was no statistically significant difference between treatment groups in terms of OS (p = 0.152) (Fig. 2). OS also showed no significant difference according to age (p = 0.162), sex (p = 0.817), tumor location (p = 0.343), grade (p = 0.257), lymphovascular invasion (p = 0.897), and nodal and TNM stage (p = 0.329, and p = 0.099, respectively). ECOG PS resulted in a significant difference in OS (p < 0.001). Patients with ECOG PS 2 had significantly lower OS than patients with PS 0 and PS 1 (p < 0.001, and p = 0.004, respectively). T stage also resulted in a significant difference in OS (p = 0.038). Patients with T4 disease had significantly lower OS than patients with T2 disease (p = 0.015). Factors associated with OS are presented in Table 2.
In subgroup analysis, DFS and OS were evaluated separately in patients with rectal cancer. In the univariant analysis, median DFS value could not be reached in mFOLFOX-6 and mFOLFOX-4 groups, while median DFS was 55.8 months in the FOLFOX-4 group. Three-year DFS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 60%, 72%, and 80%, respectively. There was a statistically significant difference between the three groups in terms of DFS (p = 0.003). The median OS value was 87.7 months in FOLFOX-4, 120 months in mFOLFOX-6, and 143 months in the mFOLFOX-4 groups. Three-year OS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 80%, 90%, and 91%, respectively. Five-year OS rates for FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups were 60%, 75%, and 73%, respectively. There was no statistically significant difference between treatment groups in terms of OS (p = 0.123) (Fig. 3).
Multivariate analyses revealed that ECOG PS (p = 0.023, and p = 0.016, respectively) and T stage (p = 0.012, and p = 0.015, respectively) were independent prognostic factors for DFS and OS. There was no statistically significant difference between the three treatment groups in terms of DFS and OS (p = 0.079, and p = 0.147, respectively) (Table 3).
Toxicity
Median number of chemotherapy cycles was 12 (6–12) for the entire population and all three treatment groups. Dose reduction was performed in 65 (48.9%), 59 (22.5%), and 11 (6.5%) patients in FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups, respectively. Dose delay was performed in 92 (69.2%), 70 (26.2%), and 30 (17.6%) patients in FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups, respectively. In patients with rectal cancer, dose reduction was performed in 23 (52.3%), 16 (20.3%), and 3 (5.9%) patients in FOLFOX-4, mFOLFOX-6, and mFOLFOX-4 groups respectively, and similarly dose delay had been needed in 32 (72.7%), 25 (50%), and 10 (20%) patients, respectively. Anemia and febrile neutropenia were not different between treatment groups (p = 0.063, and p = 0.210, respectively). Among the most common grade 3–4 adverse events, neutropenia (53.4%, 9%, and 13.5%, respectively) and diarrhea (10.5%, 2.2%, and 2.4, respectively) were more common in the FOLFOX-4 group than in the mFOLFOX-6 and mFOLFOX-4 groups. Among grade 1–2 adverse events, thrombocytopenia (24.7%, 17.3%, and 13.5%, respectively), neuropathy (37.5%, 20.3%, and 18.8%, respectively), and stomatitis (18%, 11.3%, and 10.6%, respectively) were more common in the mFOLFOX-6 group than in the FOLFOX-4 and mFOLFOX-4 groups. The frequency of grade 1-2 nausea and vomiting were similar in mFOLFOX-6 (36.3% and 24%, respectively) and mFOLFOX-4 (32.4% and 24.7%, respectively) groups and higher than FOLFOX-4 (19.5% and 11.3%, respectively) group (Table 4).
Discussion
In this study, we compared three different 5-FU administration types in FOLFOX combination treatment used after surgery in stage III colon cancer in terms of efficiency and toxicity. We demonstrated that DFS and OS did not exhibit a significant difference between the three treatment groups. The frequency of neutropenia and diarrhea, grade 3-4 adverse events important for treatment continuity, was higher in the FOLFOX-4 group than in others. However, the rates of thrombocytopenia, neuropathy, and stomatitis, which were grade 1–2 adverse events, were higher in the mFOLFOX-6 group than in the other treatment groups. The frequency of grade 1–2 nausea and vomiting was similar in the mFOLFOX-6 and mFOLFOX-4 groups, which was higher than that in the FOLFOX-4 group. To our best knowledge, because there is not enough information in the literature regarding these regime comparisons, prospective study of the mFOLFOX-4 regimen, our findings become much more valuable and attractive.
With the introduction of postoperative adjuvant chemotherapy in stage 3 colon cancer patients, significant reductions in local relapse and distant metastasis rates have been achieved. Because relapse is usually encountered in the first three years in colon cancer, studies usually report 3-year DFS rates [8]. Also, Sargent et al. reported that 3-year DFS rates were associated with 5-year OS [9].
MOSAIC and The National Surgical Adjuvant Breast and Bowel Project 07 (NSABP C07) studies are the two main randomized controlled adjuvant colon cancer studies that investigate the oxaliplatin and 5-FU/LV combination. NSABP C07 study showed that with the FLOX regimen, adding oxaliplatin to 5-FU/LV therapy yielded a 6.6% additional increase in 3-year DFS rates [6, 10]. Also, the 5-year OS rate of stage 3 patients receiving FLOX is reported to be 76.5% [10]. However, the FLOX regimen is not preferred in daily practice due to significant toxicity. MOSAIC study reported the 3-year DFS rate and 6-year OS rate to be 72.2% and 73%, respectively, in the FOLFOX-4 arm in stage 3 colon cancer. In our study, the 3-year DFS rate was 65% and the 5-year OS rate was 69% in the FOLFOX-4 group. The survival differences with the literature were due to our subjects having more poor prognostic factors than in previous studies. In our study, the ratio of patients with N2 disease (35% vs 15.1%) and poorly differentiated tumor (20% vs 12.6%) were higher than the MOSAIC study. Also, the MOSAIC study excluded patients with rectal cancer, while our study had 45 patients with rectal cancer in the FOLFOX-4 arm.
When compared with FOLFOX-4 regimen, the mFOLFOX-6 regimen is easier to administer because it only requires 1 day in the hospital, has lower costs, and is shown to have similar DFS in two phase 3 studies [11, 12]. NSABPC-08 study reported that the 3-year DFS rate was 71.7% and the 5-year OS rate was 77.6% in the mFOLFOX-6 arm of stage 3 patients [13]. In our study, consistent with the literature, the 3-year DFS rate was 72% and the 5-year OS rate was 88% in the group receiving mFOLFOX-6 therapy.
The results obtained with metastatic patients and the convenience of only one day of hospital visit made the mFOLFOX-4 regimen preferred in daily clinical practice. In a retrospective study with stage III colorectal cancer, 3-year DFS and OS rates were reported to be 65.6% and 84.3% in the mFOLFOX-4 group. In our study, we achieved a better 3-year DFS rate (72%) anda similar 3-year OS rate (83%) with the mFOLFOX-4 regimen. We were also able to achieve the 3-year DFS rate obtained with the standard FOLFOX-4 arm in the MOSAIC study (72% vs 72.2%), showing that the mFOLFOX-4 regimen is non-inferior to standard FOLFOX-4 [14].
In the subgroup analysis of patients with rectal cancer, the 3-year DFS rate for the FOLFOX-4 group was 60% and found to be lower than the other two treatment groups (p = 0.003). The local recurrence rate was also detected higher in 14.7% of patients in the FOLFOX-4 group. The shorter DFS may be due to factors that include the high positivity rate of surgical margins, 2-day bolus therapy applications that may cause a reduction in the treatment compliance with radiotherapy.
In the MOSAIC study, the most common grade 3-4 adverse events in the FOLFOX-4 arm were neutropenia with 41.1%, neuropathy with 12.4%, and diarrhea with 10.8% [6]. In our study, neutropenia was higher than the MOSAIC study with 53.4%, diarrhea was similar with 10.5%, and neuropathy was less with 3%.
In the NSABPC-08 study that evaluates adjuvant mFOLFOX-6 regimen, among grade 3-4 toxicities, neutropenia was reported to be 32.6%, thrombocytopenia 3.4%, and neuropathy 14.4% [15]. Another phase 3 study that assessed adjuvant mFOLFOX-6 regimen reported the most common grade 3-4 adverse events to be neutropenia with 26.9%, thrombocytopenia with 2.5%, neuropathy with 7.1%, and diarrhea with 4% [16]. In our study, we observed 9% grade 3-4 neutropenia, 2.6% thrombocytopenia, 2.6% neuropathy, and 2.2% diarrhea in the mFOLFOX-6 group. Grade 1-2 adverse events neuropathy (37.5%), thrombocytopenia (24.7%), and stomatitis (18%) were encountered more in the mFOLFOX-6 group than the other two treatment groups. Toxicity rates showed variations due to the retrospective nature of our study and data retrieval from patient records.
In a retrospective study that investigated the mFOLFOX-4 regimen in rectal cancer, grade 3–4 leukopenia and neuropathy were reported 9.1% and thrombocytopenia was not reported [17]. In our study, we observed more grade 3–4 neutropenia with 1.5% and less neuropathy with 4.7% in the mFOLFOX-4 arm. Thrombocytopenia was reported to be 1.2%. The number of patients receiving chemoradiotherapy was higher in the current study and the rate of neuropathy was higher due to common use of oxaliplatin.
In the adjuvant setting, the completion of all preplanned treatments after surgery is challenging due to patient tolerance and drug toxicity, and dose reduction is common oncological practice for patient management [18]. Almost all chemotherapy treatments are dosed based on body surface area (BSA) and recommended treatment doses (mg) per BSA (meters squared, m2) are derived from trials testing for dose-limiting toxicities. Body composition is one factor influencing the pharmacokinetics and metabolism of many chemotherapy agents [19]. Therefore, patients’ treatment tolerance is different, for overcoming treatment toxicity and to increase tolerance dose reduction and delays are commonly used by oncologists in their daily practice. Thereby, the maximum tolerable dose of patients can be determined. The cut-off values for dose reduction, and intensity, which will not adversely affect treatment success, are determined by adjuvant colorectal cancer studies [18, 20]. Therefore, we think, in our study, dose reductions and delays have not negatively affected the results.
The major strength of our study is including heterogenous patients from different centers and the major limitation is its retrospective nature. Also, even though certain prognostic factors were not evenly distributed, they are shown to not affect DFS and OS. Despite our limitations, our study is a large scale study that enough to separately evaluate these three different treatment arms in terms of efficiency and toxicity profile. In addition, due to the administration challenges of standard FOLFOX-4 regimen, mFOLFOX-6, and mFOLFOX-4 regimens being easy to implement into daily practice, and both regimens proving non-inferior to the standard regimen with manageably toxicity profiles in this study, our research may provide a significant contribution to the literature.
In conclusion, three chemotherapy regimens with the same doses of oxaliplatin but different 5-FU administrations can be used in daily practice for the adjuvant treatment of stage III colon cancer with similar efficiency and manageable toxicity.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics 2020. CA Cancer J for Clin 7:7–30
Fortea-Sanchis C, Forcadell-Comes E, Martinez-Ramos D et al (2019) Modelling the probability of erroneous negative lymph node staging in patients with colon cancer. Cancer Commun 39:31
O’Connell JB, Maggard MA, Ko CY (2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 96:1420–1425
Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, Veeder MH, Mailliard JA (1990) Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 322:352–358
O’Connell MJ, Laurie JA, Kahn M et al (1998) Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol 16:295–300
Andre T, Boni C, Mounedji-Boudiaf L et al (2004) Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 350:2343–2351
Andre T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27:3109–3116
Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, Mukoyama S, Yasuda S, Tajima T, Makuuchi H, Murayama C (2003) Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 50:1362–1366
Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O'Callaghan CJ, Francini G, Grothey A, O'Connell M, Catalano PJ, Blanke CD, Kerr D, Green E, Wolmark N, Andre T, Goldberg RM, de Gramont A (2005) Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23:8664–8670
Yothers G, O’Connell MJ, Allegra CJ et al (2011) Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 29:3768–3774
Allegra CJ, Yothers G, O’Connell MJ et al (2011) Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol 29:11–16
Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, Kahlenberg MS, Shields AF, Quesenberry JT, Webb TA, Farr GH Jr, Pockaj BA, Grothey A, Goldberg RM (2012) Effect of Oxaliplatin, Fluorouracil, and LeucovorinWith or Without Cetuximab on Survival Among Patients With Resected Stage III Colon Cancer. JAMA 307:1383–1393
Allegra CJ, Yothers G, O’Connell MJ et al (2013) Bevacizumab in Stage II-III Colon Cancer: 5-Year Update of the National Surgical Adjuvant Breast and Bowel Project C-08 Trial. J Clin Oncol 31:359–364
Uncu D, Aksoy S, Çetin B, Yetişyiğit T, Özdemir N, Berk V, Dane F, Inal A, Harputluoğlu H, Budakoğlu B, Koca D, Sevinç A, Cihan S, Durnalı AG, Özkan M, Öztürk MA, Işıkdoğan A, Büyükberber S, Benekli M, Köş T, Alkış N, Karaca H, Turhal NS, Zengin N, Anatolian Society of Medical Oncology (2013) Results of Adjuvant FOLFOX Regimens in Stage III Colorectal Cancer Patients: Retrospective Analysis of 667 Patients. Oncology 84:240–245
Allegra CJ, Yothers G, O’Connell MJ et al (2009) Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol 27:3385–3390
Pectasides D, Karavasilis V, Papaxoinis G et al (2015) Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer 15:384–395
Cihan Ş, Uncu D, Babacan NA, Özdemir N, Odabaş H, Aksoy S, Öksüzoğlu B, Zengin N (2011) Adjuvant modified FOLFOX-4 in patients with stage III rectum adenocarcinoma. Asian Pac J Cancer Prev 12:967–970
Aspinall SL, Good CB, ZhaoX et al (2015) Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among veterans. BMC Cancer 15:62
Cespedes Feliciano EM, Lee VS, Prado CM, Meyerhardt JA, Alexeeff S, Kroenke CH, Xiao J, Castillo AL, Caan BJ (2017) Muscle mass at the time of diagnosis of nonmetastatic colon cancer and early discontinuation of chemotherapy, delays, and dose reductions on adjuvant FOLFOX: The C-SCANS study. Cancer 123:4868–4877
Park D, Baek SJ, Kwak JM, Kim J, Kim SH (2018) Analysis of reduced-dose administration of oxaliplatin as adjuvant FOLFOX chemotherapy for colorectal cancer. Ann Surg Treat Res 94:196–202
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the local Ethics Committee of Dicle University (Approval no: 147/2019) and conducted in accordance with the Helsinki Declaration and ethical principles.
Consent for publication
All authors have approved the manuscript and consent for publication.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akdeniz, N., Kaplan, M.A., Uncu, D. et al. The comparison of FOLFOX regimens with different doses of 5-FU for the adjuvant treatment of colorectal cancer: a multicenter study. Int J Colorectal Dis 36, 1311–1319 (2021). https://doi.org/10.1007/s00384-021-03888-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-021-03888-9