Abstract
Purpose
Centralization of cancer care is expected to yield superior results. In Germany, the national strategy is based on a voluntary certification process. The effect of centre certification is difficult to prove because quality data are rarely available prior to certification. This observational study aims to assess outcomes for rectal cancer patients before and after implementation of a certified cancer centre.
Patients and methods
All consecutive patients treated for rectal cancer in our certified centre from 2009 to 2017 were retrieved from a prospective database. The dataset was analyzed according to a predefined set of 19 quality indicators comprising 36 quality goals. The results were compared to an identical cohort of patients, treated from 2000 to 2008 just before centre implementation.
Results
In total, 1059 patients were included, 481 in the 2009–2017 interval and 578 in the 2000–2008 interval. From 2009 to 2017, 25 of 36 quality goals were achieved (vs. 19/36). The proportion of anastomotic leaks in low anastomoses was improved (13.5% vs. 22.1%, p = 0.018), as was the local 5-year recurrence rate for stage (y)pIII rectal cancers (7.7% vs. 17.8%, p = 0.085), and quality of mesorectal excision (0.3% incomplete resections vs. 5.5%, p = 0.002). Furthermore, a decrease of abdominoperineal excisions was noted (47.1% vs. 60.0%, p = 0.037). For the 2009–2017 interval, local 5-year recurrence rate in stages (y)p0-III was 4.6% and 5-year overall survival was 80.2%.
Conclusions
Certification as specialized centre and regular audits were associated with an improvement of various quality parameters. The formal certification process has the potential to enhance quality of care for rectal cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2007, a German expert group published a set of quality indicators for the diagnosis and treatment of rectal cancer [1]. The group also proposed benchmarks for each indicator with a clear definition of the numerator and denominator. Thus, a set of 36 quality goals was defined. The feasibility of these quality indicators could be proven by several groups specializing in rectal cancer care [2, 3]. We were also able to give positive proof of the practicability of the quality goals for a consecutive series of patients treated for rectal cancer from 2000 to 2008 [4].
In 2008, the German Ministry of Health together with two German cancer societies and the working group of German tumour centres agreed on a national cancer plan including the strategy that patients with common cancer entities such as colorectal cancer should be treated in certified centres to ensure a high standard of care [5]. Following the instructions of this strategy, our unit was certified in May 2008 by the German Society of General and Visceral Surgery and by the German Cancer Society in 2011. Preconditions for certification were several measures of quality assurance, implementation of structured workflows, and annual external audits [6, 7]. Certification of the German Cancer Society mandated the nomination of at least two specialized colorectal cancer surgeons with a minimum of 10 rectal resections per year [7].
However, the impact of establishing certified centres for colorectal cancer on patient outcomes is difficult to assess and data that prove the benefit is scarce [8]. We hypothesized that implementation of a certified centre improves predefined quality parameters. In order to assess this, we compared the group of rectal cancer patients treated within the structure of a certified colorectal unit with a patient group treated before and documented in an identical manner [4]. The comparison was done using the detailed quality indicators of the German working group [1]. The primary endpoint of the study was the number of achieved quality goals. The secondary endpoint was the comparison of the two groups with respect to every single quality goal.
Patients and methods
Prospective tumour documentation for colorectal cancer patients was established as early as 1981 in our unit by means of a coloproctologic database. It is based on international standards [9, 10] and has been continuously improved over the years taking into account developments in diagnostics, treatment, and refinements in classification. Tumour, node, and metastasis (TNM) classification was done according to the valid edition of the International Union against Cancer (UICC) classification [11,12,13]. Follow-up is organized in a coloproctologic clinic were patients are seen annually for 5 years and biannually thereafter up to 10 years. Written informed consent for follow-up and inquiries is obtained at the outset of treatment. Further details of follow-up have been published previously [14]. According to national regulations, no formal approval of the responsible institutional review board is necessary for this kind of study.
All rectal cancer patients treated from January 2009 until December 2017 were retrieved from the database. Patients with a histologically proven primary adenocarcinoma of the rectum (≤ 16 cm from the anal verge as measured by rigid rectoscopy) were included. Carcinoma in situ was the only exclusion criterion. To provide an overview of potential confounders, we performed a comprehensive comparison of the baseline demographic, clinical, treatment, and pathological parameters. Data was analyzed according to the definitions of the German Working Group and are detailed in the respective tables [1]. Missing data are indicated with the results; the denominator was decreased accordingly. Proportions for the quality indicators are given as percentages with 95% confidence intervals (CI). Local recurrence and overall survival rates were calculated with the Kaplan–Meier method. The starting point for the estimation of local recurrences was the date of operation. However, the starting point for survival analysis was the date of diagnosis to include the time of neoadjuvant therapy. In both calculations, postoperative mortality was excluded. Postoperative mortality was defined as in-hospital death independent of the length of stay, according to the definition of the Working Group [1]. The calculation of cumulative local recurrence rates is not influenced by postoperative mortality [15]. In overall survival, exclusion of postoperative mortality reflects the tumour-related prognosis that can be achieved for the patient according to the chosen treatment [16]. The last follow-up for calculation of recurrence rates and survival analysis was set on 26 March 2019. Patients without known local recurrence or death at 5 years were censored accordingly.
This was in accordance with our previous analysis conducted for patients treated from 2000 until 2008 [4] to ensure a high comparability between the groups. Subsequently, the two groups were compared applying either the χ2 test or the Fisher exact test for categorical variables. To illustrate trends over time, we displayed annual proportions of indicators that showed a statistically significant difference with a linear regression graph fitted for each time period. The log-rank test was applied to local recurrence and survival rates. A p value of < 0.05 was considered significant. For statistical analysis, SPSS V 25 (IBM Corp., Armonk, NY) was used.
If the result for each quality goal was within the range of the predefined benchmark, the goal was counted as achieved. For indicator 5b (tumour removal clinical stages I–II), the benchmark was interpreted as ≥ 90% without upper limit, because the rationale for an upper limit was not clear. Indicator 7b (neoadjuvant treatment depending on MR-assessed infiltration of potential CRM) describes a peculiar situation and applied in our series only to patients treated within the OCUM trial [17, 18]. It was summarized in indicator 7a. If only the 95% CI comprised the benchmark, the goal was counted as nearly achieved [2]. Indicators with six or fewer patients in the denominator were not assessed for the achievement of the benchmark.
The study, although retrospective in nature, is based on prospective data and has a longitudinal character regarding the implementation of a certified centre.
Results
For the time period from 2009 until 2017, we identified 481 patients with histologically proven adenocarcinoma of the rectum (334 males, 147 females) from the database. The median age of the study population was 68 (range 36–93) years. Median follow-up was 53.0 (range 1–128) months with 23 (4.8%) patients lost to follow-up. In comparison with the 2000–2008 group, there were significantly more patients with an elevated CEA level (39.1% vs. 28.4%, p < 0.001) and with clinical stages III (53.3% vs. 34.7%, p < 0.001) and IV (26.9% vs. 16.8%, p < 0.001). The proportion of patients who did not undergo tumour resection remained stable (13.5% vs. 13.8%, p = 0.877) as did the proportion of sphincter preserving radical operations (62.1% vs. 57.3%, p = 0.169). The proportion of intersphincteric resections, however, increased significantly (6.0% vs. 2.1%, p = 0.001), while the proportion of abdominoperineal excisions (APE) decreased (13.7% vs. 17.3%, p = 0.070). Neoadjuvant therapy was administered in 57.8% vs. 43.3% (p < 0.001). Adjuvant radio(chemo)therapy almost disappeared (1.7% vs. 12.0%, p < 0.001) owing to the shift towards neoadjuvant therapy in the 2004 guidelines [19]. Pathologic parameters, especially TNM classification, were difficult to compare because of the different proportions of neoadjuvant therapy in the two time intervals. However, we observed a significant decrease of the pUICC stage I (16.1% vs. 25.5%, p = 0.001). The proportion of poorly differentiated carcinomas was significantly lower in the 2009–2017 group (15.5% vs. 23.4%, p = 0.003). In neoadjuvantly treated patients, the proportion of complete tumour regression according to Dworak [20] did not change significantly (14.2% vs. 17.2%, p = 0.389). Further patient and tumour characteristics are given in Tables 1, 2 and 3.
Overall, 25 of the 36 quality goals could be achieved in the 2009–2017 period. The set of achieved goals comprised as important goals as postoperative mortality in elective (0.8%) and emergency (14.3%) operations, pathologic CRM (only 2.5% positive), cumulative local recurrence rate at 5 years for stages (y)p0–III (4.6%) and almost all survival parameters (Tables 4, 5, 6 and 7). The 5-year overall survival for stages (y)p0–III was 80.2%.
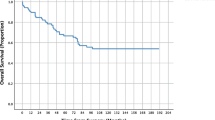
In the 2009–2017 group, 6 more quality goals could be achieved than in the 2000–2008 group. Amongst the improved quality indicators are the proportion of anastomotic leaks in anastomoses up to 7 cm from the anal verge (13.5% vs. 22.1%, p = 0.018) and the local recurrence rate of stage (y)pIII rectal cancers (7.7% vs. 17.8%, p = 0.085; Fig. 1). Furthermore, the quality of mesorectal excision improved significantly (0.3% incomplete resections vs. 5.5%, p = 0.002). For the proportion of APE in rectal cancers of the lower third, no benchmark was defined, but we could show a significant decrease from 60.0% to 47.1%, p = 0.037. Trends over time are displayed for proportions of both anastomotic leaks and APE in Figs. 2 and 3.
Another 5 quality goals were nearly achieved and only 4 quality goals were missed in 2009–2017. Missed goals included the proportion of specimens with ≥ 12 retrieved lymph nodes. Whereas the proportion remained stable for patients without neoadjuvant radio(chemo)therapy (76.6% vs. 76.4% in 2000–2008), it improved for patients after neoadjuvant therapy (58.2% vs. 45.7%, p = 0.120). We could not detect differences in overall survival, neither in the 2009–2017 group (73.3% for < 12 lymph nodes vs. 78.2% for ≥ 12 lymph nodes, p = 0.306), nor in the 2000–2008 group (69.6% for < 12 lymph nodes vs. 73.8% for ≥ 12 lymph nodes, p = 0.581).
Overall, the quality of documentation increased with the quality of mesorectal excision and pathologic CRM completely documented and urinary catheter newly documented in the 2009–2017 period. In the 2000–2008 group, three goals could not be evaluated; in the current group, this applied to two goals.
Discussion
Our study shows that despite starting from a high level, further improvements in the quality of rectal cancer treatment are possible with the implementation of a certified centre. Improved key indicators were the local recurrence rate for stage (y)pIII tumours and the anastomotic leak rate in low anastomoses. Furthermore, we could decrease the proportion of abdominoperineal excisions.
The reasons for improvement are complex and multifactorial, with causal factors for singular developments difficult to specify. However, certification as a centre necessitates the implementation of a whole bundle of measures aimed at improving the structure, process and outcome quality. These measures include the regular discussion of all rectal cancer patients in the multidisciplinary team (MDT), quality meetings at a regular base and annual audits [7, 23,24,25]. Furthermore, every elective operation must be done or supervised by a specialized surgeon, and a certain percentage of patients must be included in registered clinical studies.
Local recurrence
Local recurrence is the key quality indicator of rectal cancer treatment with major improvements within the last decades. The most important step towards an overall local recurrence rate of below 10% was the embryology-based surgical technique with the introduction of total mesorectal excision [26, 27]. This approach was supplemented by extralevator abdominoperineal excision (ELAPE) for very low-lying tumours [28,29,30]. Both steps were flanked by a critical pathological work-up of the specimens resulting in measurements of the CRM and specimen grading [31,32,33]. Additionally, the assessment of the tumour in relation to the potential CRM became available with improved MRI techniques [34,35,36]. These diagnostic measures gave a stimulating feedback to the surgeon. The nomination of specialized surgeons very likely accelerated the improvement of surgical quality [37,38,39]. Furthermore, multidisciplinary management with (neoadjuvant) radio(chemo)therapy was able to further halve local recurrence rates [40, 41].
The low local recurrence rate for all tumours in our series reflects the implementation of these developments. In particular, the improvement of local recurrences in stage (y)pIII tumours might be a result of regular discussion at our MDT with a tailored approach to these advanced tumours [42]. In the high-risk group, however, this positive trend was offset by a rather high local recurrence rate in stage (y)pII. Local recurrence in the latter stage depends heavily on the proportion of (y)pT4N0 to (y)pT3N0 tumours. In our series, the percentage of (y)pT4N0 tumours was double as high (7.9%) in 2009–2017 than in 2000–2008 (3.8%).
Overall, our recurrence rates compare favourably with the literature (cumulative 5-year local recurrence rate for stages (y)p0–III 4.6% (2009–2017 period) in our series compared with 3.5–10.6% [2, 3, 43]).
Anastomotic leak
The rather high anastomotic leak rate in our previous study induced a permanent discussion at the quality meetings with the successive implementation of measures that lead to stepwise improvement of the leak rate. Amongst these measures were the training of junior surgeons in the handling of stapling devices and the introduction of oral antibiotics in preoperative bowel preparation. Again, the increased case load of the specialized surgeons may also have contributed to the success. Reduction of anastomotic leak rates has been reported by various groups that analyzed the benefit of surgical specialization [44, 45].
Proportion APE
A further observation of our study is that the proportion of APE for tumours of the lower third of the rectum could significantly be reduced from 60.0 to 47.1%. Many factors have contributed to this development as the better MRI assessment of tumours of the lower third with high-resolution MRI and criteria according to the Low Rectal Cancer (MERCURYII) study [36] and surgical specialization [38, 46, 47]. Apart from surgical improvements and the oncologic feasibility, there are nowadays functional scores at hand that allow a proper counselling of patients in the decision-making process if an ultralow anterior/intersphincteric resection is discussed against an APE [48]. In our patient cohort, only very few patients declared that a permanent stoma would have been the better choice indicating an appropriate patient selection [49].
Pathologic CRM
The most important surrogate prognostic parameter is the free pathologic CRM [32]. It reflects various parameters of process quality such as exact MRI assessment of the primary tumour (and its lymph nodes) in relation to the mesorectal fascia, the correct decision-making of the MDT with respect to neoadjuvant therapy, the quality of re-assessment after neoadjuvant therapy and the quality of surgery including strategies to extend the operation in areas where the mesorectal fascia is infiltrated [50,51,52,53]. It strongly correlates to local recurrence and was chosen as the study endpoint in many important studies [36, 54,55,56]. Recent studies have reported a pathologic CRM involvement in 5.4 to 10% [51, 57, 58]. In our series, it improved from 5.2 to 2.5% and was in both time periods well below the target value of < 10%.
Survival
Survival data also improved favourably. Especially in stages (y)pIII, overall survival at 5 years improved from 60.7 to 69.4% and was well beyond the benchmark of 55%. The reasons for this development are similar to those given for the improvement of local recurrence rates [59, 60]. Furthermore, trends as advanced liver surgery, peritoneal surface surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), the availability of targeted therapy, radiofrequency ablation (RFA) and selective intracorporal radiotherapy (SIRT) have contributed to improved survival in those patients who develop metachronous metastases. The benchmark was only nearly achieved for stages (y)p0–I. The cause for the latter is not quite clear; only 3 of the 13 events in the 2009–2017 group (n = 133) were tumour-related.
Urinary function
The newly documented quality indicator was urinary function. Data that would allow a reasonable benchmark are rare in the literature. We noticed that 6.8% of patients were discharged with a permanent urinary catheter (suprapubic and urethral combined), which is well below 10%. Merkel et al. reported a proportion of 5.8% in the most recent patient group of their study [2]. Therefore, the proposed target value seems to be reasonable; however, it does not take T4 tumours with the possible need to remove the pelvic side wall compartment and the ensuing inevitable damage to autonomic nerves into account.
Retrieved lymph nodes
The number of retrieved lymph nodes was amongst the quality goals that were not achieved. While the proportion of patients with ≥ 12 investigated lymph nodes improved considerably in patients after neoadjuvant radio(chemo)therapy, it remained stable for patients without neoadjuvant treatment. Lymph node detection is an indicator of both surgical and pathological quality. As a direct effect of external auditing and measures taken to enhance the number of retrieved lymph nodes, we could indeed detect a continuous improvement in the last few years; however, for the entire 2009–2017 period, it was not sufficient for an achievement or near achievement of the goal.
A number of studies indicate that lymph node retrieval is a prognostic factor with respect to survival [61,62,63,64]. There is, however, some controversy in the literature as to the threshold of examined lymph nodes, which was given at a range of 8–9 in some studies [65,66,67]. The impact of lymph node yield after neoadjuvant CRT is also not yet clear, because preoperative treatment may well result in complete disappearance of lymph nodes [68,69,70]. For ypN0 cancers, no correlation of survival with number of retrieved lymph nodes was found [67, 71, 72]. We could not detect a significant difference in survival for patients with less than 12 examined lymph nodes compared to the group with ≥ 12 nodes in our series. It may well be that a stable surgical quality can compensate the drawbacks that might result from a limited lymph node retrieval [73,74,75].
The strength of our study is the completeness and quality of our database, which enabled us to analyze our quality parameters shortly after the set of quality indicators was published in 2007. The availability of a level 3 guideline and its full implementation in daily clinical practice [19] coincided with the certification of our unit as a coloproctologic/bowel cancer centre. Thus, phase 2 of the study reflects the adherence to guidelines and the results achievable with this strategy. The implementation of a certified centre in 2008 can be seen as an “intervention,” and the following time period mirrors the effect of it. There are only minor changes in the two patient groups as reflected by the demographic data and a number of quality indicators that characterize the composition of the groups. A direct comparison of the two groups which comprise more than 1000 patients with rectal cancer is therefore warranted and shows an improvement in four quality goals (11.1%) that could now be achieved. Another two quality goals that were not documented before or not applicable were now additionally achieved. This observation adds to the growing body of evidence that treatment of patients within centres improves quality of care [76,77,78].
The German Cancer Society implemented its own set of quality indicators and benchmarks for annual reporting at the external audits. Likewise, quality indicators were defined within the German Guidelines Colorectal Cancer [79]. Both sets of quality indicators underwent a process of refining over the years with changing items and target values. The 31 quality indicators defined by the German Cancer Society in 2018 reflected only five indicators as defined by the Working Group and used herein. The local recurrence rate at 5 years is not reported. The most recent version of the German Guidelines (released in 2019) defines only 12 quality indicators without target values. There are several reasons for these differences including the coverage of quality indicators that only apply to colon cancer, the definition of more general goals and the function of quality indicators as control instruments. To provide a comprehensive report on rectal carcinoma treatment outcome, we esteem the quality indicators as published by the working group as the most detailed set of parameters available so far. Nevertheless, some quality indicators may need to be reconsidered, such as the R1,2 rate of < 20% or the inclusion of the distance of the tumour to the mesorectal fascia in pretherapeutic MRI. It is, however, beyond the scope of this study to provide suggestions for change, because for that a formal consensus process based on a meta-analysis for each item would be needed.
While the German approach to higher specialization is via voluntary certification, there are some European countries that implemented centralization by administration and auditing on a national/regional level. Reports from these countries, namely Sweden, Norway, Netherlands and Spain (Catalonia), show a permanent improvement in a number of quality indicators, especially regarding survival, local recurrence, negative CRM, use of neoadjuvant treatment, discussion at MDTs and mortality [78, 80,81,82,83]. For local recurrence, a decrease at 5 years on a national level from 8.7 to 5.0% was recently reported from Sweden [80] and from 14.5 to 5.0% from Norway [83]. A voluntary training program in Spain also resulted in a 5-year local recurrence rate of 4.7% [84].
While these data underline the benefit of centralization, it has recently been questioned with respect to German cancer centres. Vogel did not find factors associated with anastomotic leak reflected in the subset of structure and process parameters of the data entry form [85]. Likewise, Ghadban et al. could not detect improvements in several aspects of morbidity on a national level despite the annual numeric increase of certified bowel cancer centres [86]. However, they used administrative data which were not based on a dedicated cancer registry or structured audit. The quality of these data is therefore questionable [87].
Our study reveals some limitations that need to be discussed. First, although based on prospectively collected data, the study is retrospective in nature. However, the inclusion of all consecutive patients, the high follow-up rate and the assessment by means of predefined quality indicators render the results valid for general conclusions. Second, during the study period, four editions of the TNM classification were in operation. Apart from a number of subclassifications which were of no relevance for our study, there were changes regarding the classification of small perirectal tumour deposits. Whereas these deposits were classified as T3 if smaller than 3 mm (5th ed.) or if irregular in shape (6th ed.), they were classified as lymph nodes (N1c) in the 7th and 8th editions [11,12,13, 21]. Therefore, some patients were classified N positive in the recent group with the resulting stage migration. The magnitude of affected patients, however, is only in the range of 1% (2 patients out of 317 resected patients without distant metastases). Third, the complexity of the analyses and the long time period make the study liable for confounding. Whereas we displayed patient, treatment and pathological characteristics of the two cohorts and used the predefined numerators and denominators to maximize comparability, additional potential confounders may bias the results. These includes more detailed characteristics that were not included in the database like performance status or co-morbidities, as well as diagnostic and treatment measures that evolved over time independently of the centralization of patient care. The latter include a more comprehensive staging by high-resolution MRI, refined surgical techniques as ELAPE, the introduction of new strategies in the management of metachronous metastases and the start of a screening colonoscopy program in 2002. However, the implementation of these new developments is likely to be accelerated by the multidisciplinary teamwork of a certified centre.
Conclusion
Implementation of clinical pathways within a certified centre is associated with an improvement in the quality of care for rectal cancer patients. Using a set of 36 predefined quality goals, six more quality goals could be achieved as compared with a previous study period. Improvement was detected in complex indicators such as the local recurrence rate in stage (y)pIII patients, anastomotic leak rate in low anastomoses and the APE rate for low rectal cancers. Certification as a specialized centre therefore signals high standard of care.
References
Bittner R, Burghardt J, Gross E, Grundmann R, Hermanek P, Isbert C, Junginger T, Köckerling F, Merkel S, Möslein G, Raab HR, Roder J, Ruf G, Schwenk W, Strassburg J, Tannapfel A, de Vries A, Zühlke H (2007) Quality indicators for diagnostic and therapy of rectal carcinoma. Zentralbl Chir 132:85–94 [in German]
Merkel S, Klossek D, Göhl J, Papadopoulos T, Hohenberger W, Hermanek P (2009) Quality management in rectal carcinoma: what is feasible ? Int J Color Dis 24:931–942
Ruppert R, Ptok H, Strassburg J et al (2013) Quality indicators of diagnosis and therapy in MRI-based neoadjuvant radiochemotherapy for rectal cancer - interim analysis of a Prospective Multicentre Observational Study (OCUM). Zentralbl Chir 138:630–635 [in German]
Stelzner S, Hellmich G, Haroske G, Puffer E, Jackisch T, Witzigmann H (2010) Practicability of quality goals for the treatment of rectal cancer. Int J Color Dis 25:1093–1102
German Ministry of Health (2012) National Cancer Plan. https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Praevention/Broschueren/Broschuere_Nationaler_Krebsplan.pdf. Accessed 22 March 2020 [in German]
German Society of General and Visceral Surgery (2016) Instructions for certification. https://www.dgav.de/zertifizierung/zertifizierungsordnung.html. Accessed 22 March 2020 [in German]
DKG (German Cancer Society) (2019) Certification Committee for Colorectal Cancer Centres. Catalogue of requirements for colorectal cancer centres. https://www.onkozert.de/organ/darm/. Accessed 22 March 2020
Völkel V, Draeger T, Gerken M, Fürst A, Klinkhammer-Schalke M (2019) Long-term survival of patients with colon and rectum carcinomas: is there a difference between cancer centers and non-certified hospitals? Gesundheitswesen 81:801–807 [in German]
Hermanek P, Henson DE, Hutter RVP, Sobin LH (1993) TNM supplement. A commentary on uniform use. Springer Berlin, Heidelberg, New York
Fielding LP, Arsenault PA, Chapuis PH et al (1991) Clinicopathological staging for colorectal cancer. An International Documentation System (IDS) and an International comprehensive Anatomical Terminology (ICAT). J Gastroenterol Hepatol 6:3225–3244
Sobin LH, Wittekind C (eds) (1997) TNM classification of malignant tumours, 5th edn. J. Wiley & Sons, New York
Sobin LH, Wittekind C (eds) (2002) TNM classification of malignant tumours, 6th edn. J. Wiley & Sons, New York
Sobin LH, Gospodarowicz MK, Wittekind C (eds) (2010) TNM classification of malignant tumours, 7th edn. J. Wiley & Sons, New York
Fischer J, Hellmich G, Jackisch T, Puffer E, Zimmer J, Bleyl D, Kittner T, Witzigmann H, Stelzner S (2015) Outcome for stage II and III rectal and colon cancer equally good after treatment improvement over three decades. Int J Color Dis 30:797–806
Merkel S, Mansmann U, Hohenberger W, Hermanek P (2006) Uniform calculation of local recurrence rates – requirement for quality management in rectal carcinoma. Z Arztl Fortbild Qualitatssich 100:183–187 [in German]
Hermanek P, Mansmann U (2001) Criteria for assessment of prognostic factors. Chirurg 72:474–480 [in German]
Ruppert R, Junginger T, Ptok H, Strassburg J, Maurer CA, Brosi P, Sauer J, Baral J, Kreis M, Wollschlaeger D, Hermanek P, Merkel S, the OCUM group (2018) Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM Rectal Cancer Trial. Br J Surg 105:1519–1529
Kreis ME, Ruppert R, Kube R et al (2020) MRI-based use of neoadjuvant chemoradiotherapy in rectal carcinoma: surgical quality and histopathological outcome of the OCUM trial. Ann Surg Oncol 27:417–427
Schmiegel W, Pox C, Adler G et al (2004) S3-guideline conference “Colorectal Cancer” 2004. Z Gastroenterol 42:1129–1177 [in German]
Dworak O, Keilholz L, Hoffmann A (1997) Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Color Dis 12:19–23
Brierley JD, Gospodarowicz MK, Wittekind C (eds) (2017) TNM classification of malignant tumours, 8th edn. J. Wiley & Sons, New York
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351
Kowalski C, Graeven U, von Kalle C, Lang H, Beckmann MW, Blohmer JU, Burchardt M, Ehrenfeld M, Fichtner J, Grabbe S, Hoffmann H, Iro H, Post S, Scharl A, Schlegel U, Seufferlein T, Stummer W, Ukena D, Ferencz J, Wesselmann S (2017) Shifting cancer care towards multidisciplinarity: the cancer center certification program of the German cancer society. BMC Cancer 17:850
Prades J, Manchon-Walsh P, Solà J, Espinàs JA, Guarga A, Borras JM (2016) Improving clinical outcomes through centralization of rectal cancer surgery and clinical audit: a mixed-methods assessment. Eur J Pub Health 26:538–542
van de Velde CJ, van den Broek CB (2012) Quality assurance in rectal cancer treatment. Dig Dis 30(Suppl 2):126–131
Heald RJ, Husband EM, Ryall RDH (1982) The mesorectum in rectal cancer surgery – the clue to pelvic recurrence? Br J Surg 69:613–616
Martling A, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedermark B (2000) Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Lancet 356:93–96
Holm T, Ljung A, Häggmark T, Jurell G, Lagergren J (2007) Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg 94:232–238
Palmer G, Anderin C, Martling A, Holm T (2014) Local control and survival after extralevator abdominoperineal excision for locally advanced or low rectal cancer. Color Dis 16:527–532
Stelzner S, Hellmich G, Sims A, Kittner T, Puffer E, Zimmer J, Bleyl D, Witzigmann H (2016) Long-term outcome of extralevator abdominoperineal excision (ELAPE) for low rectal cancer. Int J Color Dis 31:1729–1737
Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2:996–999
Nagtegaal ID, Quirke P (2008) What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 26:303–312
Maughan NJ, Quirke P (2003) Modern management of colorectal cancer – a pathologist’s view. Scand J Surg 92:11–19
Mercury Study Group (2006) Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal adenocarcinoma: prospective observational study. BMJ 333:749–782
Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M (2005) Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol 78:245–251
Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, Strassburg J, Quirke P, Tekkis P, Pedersen BG, Gudgeon M, Heald B, Brown G (2016) Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II study. Ann Surg 263:751–760
Hermanek P (1999) Impact of surgeon’s technique on outcome after treatment of rectal carcinoma. Dis Colon Rectum 42:559–562
Archampong D, Borowski D, Wille-Jørgensen P, Iversen LH (2012) Workload and surgeon's specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005391
Machado M, Goldman S, Järhult J (2000) Improved results in rectal cancer surgery – an effect of specialization? Color Dis 2:264–269
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken J, Leer JW, van de Velde C, Dutch Colorectal Cancer Group (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Palmer G, Martling A, Cedermark B, Holm T (2011) Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Color Dis 13:1361–1369
Heald RJ, Moran BJ, Ryall RDH, Sexton R, MacFarlane JK (1998) The Basingstoke Experience of total mesorectal excision, 1978 – 1997. Arch Surg 133:894–899
Roxburgh CSD, Strombom P, Lynn P, Cercek A, Gonen M, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Shia J, Vakiani E, Yaeger R, Stadler ZK, Segal NH, Reidy D, Varghese A, Wu AJ, Crane CH, Gollub MJ, Saltz LB, Garcia-Aguilar J, Weiser MR (2019) Changes in the multidisciplinary management of rectal cancer from 2009 to 2015 and associated improvements in short-term outcomes. Color Dis 21:1140–1150
García-Granero E, Navarro F, Cerdán Santacruz C, Frasson M, García-Granero A, Marinello F, Flor-Lorente B, Espí A (2017) Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: an institutional analysis of 800 patients. Surgery 162:1006–1016
Iversen LH, Harling H, Laurberg S, Wille-Jørgensen P (2007) Influence of caseload and surgical speciality on outcome following surgery for colorectal cancer: a review of evidence. Part 2: long-term outcome. Color Dis 9:38–46
Borowski DW, Kelly SB, Bradburn DM, Wilson RG, Gunn A, Ratcliffe AA (2007) Impact of surgeon volume and specialization on short-term outcomes in colorectal cancer surgery. Br J Surg 94:880–889
Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, Christensen P, Laurberg S, Moran BJ, UK and Danish LARS Study Groups (2017) Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut 67:688–696
Kupsch J, Jackisch T, Matzel KE, Zimmer J, Schreiber A, Sims A, Witzigmann H, Stelzner S (2018) Outcome of bowel function following anterior resection for rectal cancer– an analysis using the Low Anterior Resection Syndrome (LARS) score. Int J Color Dis 33:787–798
Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D (2006) MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 94:351–357
Wiegering A, Buhr HJ, Klinger C et al (2018) Quality indicators for surgery of rectal cancer : evidence-based development of a set of indicators for quality. Chirurg. 89:26–31 [in German]
Massarweh NN, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Cormier JN, Feig BW, Chang GJ (2014) Risk-adjusted pathologic margin positivity rate as a quality indicator in rectal cancer surgery. J Clin Oncol 32:2967–2974
Justiniano CF, Aquina CT, Fleming FJ, Xu Z, Boscoe FP, Schymura MJ, Temple LK, Becerra AZ (2019) Hospital and surgeon variation in positive circumferential resection margin among rectal cancer patients. Am J Surg 218:881–886
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas M, de Lange-de Klerk ES, Lacy AM, Bemelman WA, Andersson J, Angenete E, Rosenberg J, Fuerst A, Haglind E, COLOR II Study Group (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332
Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PWT, Nelson H (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA 314:1346–1355
Bianco F, Romano G, Tsarkov P, Stanojevic G, Shroyer K, Giuratrabocchetta S, Bergamaschi R, for the International Rectal Cancer Study Group (2017) Extralevator with vs nonextralevator abdominoperineal excision for rectal cancer: the RELAPe randomized controlled trial. Color Dis 19:148–157
Detering R, Saraste D, de Neree Tot Babberich MPM et al (2020) International evaluation of circumferential resection margins after rectal cancer resection: insights from the Swedish and Dutch audits. Color Dis 22:416–429
Warrier SK, Kong JC, Guerra GR, Chittleborough TJ, Naik A, Ramsay RG, Lynch AC, Heriot AG (2018) Risk factors associated with circumferential resection margin positivity in rectal cancer: a binational registry study. Dis Colon Rectum 61:433–440
Hohenberger W, Merkel S, Hermanek P (2013) Volume and outcome in rectal cancer surgery: the importance of quality management. Int J Color Dis 28:197–206
Etzioni DA, Young-Fadok TM, Cima RR, Wasif N, Madoff RD, Naessens JM, Habermann EB (2014) Patient survival after surgical treatment of rectal cancer: impact of surgeon and hospital characteristics. Cancer 120:2472–2481
Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB III, Cummings B, Gunderson L, Macdonald JS, Mayer RJ (2001) Impact of number of lymph nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 19:157–163
Kim YW, Kim NK, Min BS, Lee KY, Sohn SK, Cho CH (2009) The influence of the number of retrieved lymph nodes on staging and survival in patients with stage II and III rectal cancer undergoing tumor-specific mesorectal excision. Ann Surg 249:965–972
Cox ML, Adam MA, Shenoi MM, Turner MC, Sun Z, Mantyh CR, Migaly J (2018) Resected irradiated rectal cancers: are twelve lymph nodes really necessary in the era of neoadjuvant therapy? Am J Surg 216:444–449
Xu Z, Berho ME, Becerra AZ, Aquina CT, Hensley BJ, Arsalanizadeh R, Noyes K, Monson JRT, Fleming FJ (2017) Lymph node yield is an independent predictor of survival in rectal cancer regardless of receipt of neoadjuvant therapy. J Clin Pathol 70:584–592
Hall MD, Schultheiss TE, Smith DD et al (2015) Impact of total lymph node count on staging and survival after neoadjuvant chemoradiation therapy for rectal cancer. Ann Surg Oncol 22(Suppl 3):S580–S587
Raoof M, Nelson RA, Nfonsam VN, Warneke J, Krouse RS (2016) Prognostic significance of lymph node yield in ypN0 rectal cancer. Br J Surg 103:1731–1737
Gill A, Brunson A, Lara PJ, Khatri V, Semrad TJ (2015) Implications of lymph node retrieval in locoregional rectal cancer treated with chemoradiotherapy: a California Cancer Registry Study. Eur J Surg Oncol 41:647–652
Habr-Gama A, Perez RO, Proscurshim I, Rawet V, Pereira DD, Sousa AHS, Kiss D, Cecconello I (2008) Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: what does it mean? Dis Colon Rectum 51:277–283
Kim HJ, Jo JS, Lee SY, Kim CH, Kim YJ, Kim HR (2015) Low lymph node retrieval after preoperative chemoradiation for rectal cancer is associated with improved prognosis in patients with a good tumor response. Ann Surg Oncol 22:2075–2081
Bustamante-Lopez L, Nahas CS, Nahas SC, Ribeiro U Jr, Marques CF, Cotti G, Rocco A, Cecconello I (2017) Understanding the factors associated with reduction in the number of lymph nodes in rectal cancer patients treated by neoadjuvant treatment. Int J Color Dis 32:925–927
Abdel-Misih SR, Wei L, Benson AB 3rd et al (2016) Neoadjuvant therapy for rectal cancer affects lymph node yield and status without clear implications on outcome: the case for eliminating a metric and using preoperative staging to guide therapy. J Natl Compr Cancer Netw 14:1528–1534
Degiuli M, Arolfo S, Evangelista A, Lorenzon L, Reddavid R, Staudacher C, de Nardi P, Rosati R, Elmore U, Coco C, Rizzo G, Belluco C, Forlin M, Milone M, de Palma GD, Rega D, Delrio P, Guerrieri M, Ortenzi M, Muratore A, Marsanic P, Restivo A, Deidda S, Zuin M, Pucciarelli S, de Luca R, Persiani R, Biondi A, Roviello F, Marrelli D, Sgroi G, Turati L, Morino M (2018) Number of lymph nodes assessed has no prognostic impact in node-negative rectal cancers after neoadjuvant therapy. Results of the “Italian Society of Surgical Oncology (S.I.C.O.) Colorectal Cancer Network” (SICO-CCN) multicntre collaborative study. Eur J Surg Oncol 44:1233–1240
Raoof M, Zafar SN, Ituarte PHG, Krouse RS, Melstrom K (2019) Using a lymph node count metric to identify underperforming hospitals after rectal cancer surgery. J Surg Res 236:216–223
Chand M, Moran BJ, Jones RG, Heald RJ, Brown G (2014) Lymph node status does not predict local recurrence in the total mesorectal excision era. Dis Colon Rectum 57:127–129
Leonard D, Remue C, Abbes Orabi N, van Maanen A, Danse E, Dragean A, Debetancourt D, Humblet Y, Jouret-Mourin A, Maddalena F, Medina Benites A, Scalliet P, Sempoux C, van den Eynde M, de Schoutheete JC, Kartheuser A (2016) Lymph node ratio and surgical quality are strong prognostic factors of rectal cancer: results from a single referral centre. Color Dis 18:O175–O184
Weinhold I, Keck T, Merseburger A et al (2018) Utility analysis of oncological centre building in the field of colorectal cancer. Zentralbl Chir 143:181–192 [in German]
Wesselmann S, Winter A, Ferencz J, Seufferlein T, Post S (2014) Documented quality of care in certified colorectal cancer centers in Germany: German Cancer Society benchmarking report for 2013. Int J Color Dis 29:511–518
Manchon-Walsh P, Aliste L, Espinàs JA, Prades J, Guarga A, Balart J, Biondo S, Castells A, Sanjuan X, Tabernero J, Borras JM, Biondo S, Cambray M, Castells A, Codina A, Espín E, Musulen E, Pozuelo A, Saigi E, Sala J, Salas A, Salazar R, Sanjuán X, Tabernero J, Targarona EM (2016) Improving survival and local control in rectal cancer in Catalonia (Spain) in the context of centralisation: a full cycle audit assessment. Eur J Surg Oncol 42:1873–1880
Guideline Programme Oncology (2019) S3-Guideline colorectal cancer, version 2.1, http://www.leitlinienprogramm-onkologie.de/leitlinen/kolorektales-karzinom/. Accessed 17 July 2020 [in German]
Kodeda K, Johansson R, Zar N, Birgisson H, Dahlberg M, Skullman S, Lindmark G, Glimelius B, Påhlman L, Martling A (2015) Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Color Dis 17:O168–O179
van Leersum NJ, Snijders HS, Henneman D, Kolfschoten NE, Gooiker GA, ten Berge M, Eddes EH, Wouters MW, Tollenaar RA, Dutch Surgical Colorectal Cancer Audit Group, Bemelman WA, van Dam R, Elferink MA, Karsten TM, van Krieken J, Lemmens VE, Rutten HJ, Manusama ER, van de Velde C, Meijerink WJ, Wiggers T, van der Harst E, Dekker JW, Boerma D (2013) The Dutch surgical colorectal audit. Eur J Surg Oncol 39:1063–1070
Gietelink L, Henneman D, van Leersum NJ et al (2016) The influence of hospital volume on circumferential resection margin involvement: results of the Dutch surgical colorectal audit. Ann Surg 263:745–750
Guren MG, Kørner H, Pfeffer F, Myklebust TÅ, Eriksen MT, Edna TH, Larsen SG, Knudsen KO, Nesbakken A, Wasmuth HH, Vonen B, Hofsli E, Færden AE, Brændengen M, Dahl O, Steigen SE, Johansen MJ, Lindsetmo RO, Drolsum A, Tollåli G, Dørum LM, Møller B, Wibe A (2015) Nationwide improvement of rectal cancer treatment outcomes in Norway, 1993-2010. Acta Oncol 54:1714–1722
Ortiz H, Wibe A, Ciga MA, Lujan J, Codina A, Biondo S (2013) Impact of a multidisciplinary team training programme on rectal cancer outcomes in Spain. Color Dis 15:544–551
Vogel PA (2019) Why certification of colorectal cancer centres does not improve surgical quality. Zentralbl Chir 144:273–280 [in German]
Ghadban T, Reeh M, Bockhorn M, Grotelueschen R, Bachmann K, Grupp K, Uzunoglu FG, Izbicki JR, Perez DR (2019) Decentralized colorectal cancer care in Germany over the last decade is associated with high in-hospital morbidity and mortality. Cancer Manag Res 11:2101–2107
Corbellini C, Andreoni B, Ansaloni L, Sgroi G, Martinotti M, Scandroglio I, Carzaniga P, Longoni M, Foschi D, Dionigi P, Morandi E, Agnello M, Lombardy Oncologic Network Work Group (2018) Reliability and validity assessment of administrative databases in measuring the quality of rectal cancer management. Tumori 104:51–59
Acknowledgements
The authors are indebted to Dr. René Mauer for his statistical advise and to Lisa Domichowski and Anja Willing, Medical Data Managers, for their support in data acquisition.
Funding
The maintenance of the database at the Coloproctologic Unit of Dresden-Friedrichstadt General Hospital is supported by a grant from the Tumour Centre Dresden.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacob, A., Albert, W., Jackisch, T. et al. Association of certification, improved quality and better oncological outcomes for rectal cancer in a specialized colorectal unit. Int J Colorectal Dis 36, 517–533 (2021). https://doi.org/10.1007/s00384-020-03792-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03792-8