Abstract
Purpose
This study aimed to investigate the association between metformin usage and the risk of colorectal cancer (CRC) using data from the Korean National Health Insurance Service–National Health Screening Cohort database.
Methods
Data from the NHIS-HEALS cohort between 2002 and 2015 were longitudinally analyzed. Subjects were divided into three groups: metformin non-users with diabetes mellitus (DM), metformin users with DM, and no DM group. CRC was defined using the ICD-10 code (C18.0-C20.0) at the time of admission. Cox proportional hazard regression models were adopted after stepwise adjustment for confounders to investigate the association between metformin usage and colorectal cancer risk.
Results
During the follow-up period, of the total 323,430 participants, 2341 (1.33%) of the 175,495 males and 1204 (0.81%) of the 147,935 females were newly diagnosed with CRC. The estimated cumulative incidence of CRC was significantly different among the three groups based on Kaplan-Meier’s survival curve (p values < 0.05 in both sexes). Compared with metformin non-users, hazard ratios (95% CIs) of metformin users and the no DM group were 0.66 (0.51–0.85) and 0.72 (0.61–0.85) in males and 0.59 (0.37–0.92) and 0.93 (0.66–1.29) in females, respectively, after being fully adjusted.
Conclusions
Metformin users with diabetes appear to have a significantly lower risk of CRC compared with metformin non-users.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second cause of cancer deaths worldwide, accounting for over 1.8 million new cases and about 880,000 deaths in 2018 [1]. The incidence and mortality rates due to CRC are increasing in many low-income and middle-income countries [2]. In Korea, malignant neoplasm accounts for one in four deaths and over 220,000 cases were newly diagnosed in 2016 [3]. The age-standardized incidence and mortality of CRC in Korea were 30.7 and 8.2 per 100,000 persons in 2016, respectively [3].
The best way to reduce cancer mortality is prevention and early detection. Health authorities in Korea provide a national cancer screening program for the five most common cancer types, which involve the stomach, liver, colorectum, breast, and uterine cervix. Early diagnosis of CRC may contribute to better survivor outcomes and quality of life. Some agents are known to have a preventive effect on colorectal carcinogenesis. Nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, are drugs that have been reported in many studies as chemopreventive agents against CRC [4,5,6]. The US Preventive Services Task Force (USPSTF) recommended the use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) and CRC in adults aged 50 to 59 years [7].
Metformin is the first treatment option with lifestyle modification for type 2 diabetes mellitus (DM) management [8]. Additionally, it has recently attracted attention as a chemopreventive agent of CRC development along with aspirin or NSAIDs [9,10,11,12]. The main action of metformin in lowering blood glucose levels is the inhibition of gluconeogenesis in the liver and to enhance insulin sensitivity in peripheral tissue [13]. In addition to glycemic control, recent previous studies have reported that it may have pleiotropic effects such as carcinogenesis prevention and immune modulation [14,15,16]. Metformin may be mediated through the activation of adenosine monophosphate (AMP)–activated protein kinase (AMPK) at a molecular level [13, 16].
This study aimed to investigate the association between metformin usage and the prevalence of malignant neoplasm of the colorectum using the Korean National Health Insurance Service (NHIS)–National Health Screening (NHIS-HEALS) cohort after adjusting for age and other confounding factors including socioeconomic status, health behaviors, and laboratory data.
Methods
Data source and study population
This retrospective population-based cohort study was performed using the NHIS-HEALS cohort database in Korea. The database contains information on 514,794 people, 10% of the 5.15 million people who are between the ages of 40 and 79 as of December 2002, and who had a health examination between 2002 and 2003. The information included in the database is age, sex, laboratory findings, medical records (including diagnostic codes and prescription data), socioeconomic status, death information, and health behaviors obtained from self-reported questionnaires between 2002 and 2015. A detailed description of the study design and methods was published previously [17].
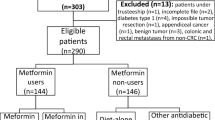
Figure 1 is a flowchart that shows how participants were selected for the analysis. Because elderly participants have a higher probability of being censored due to death or loss to follow-up during the study period, we first excluded people over 70 years of age between 2002 and 2003 (n = 38,519). Additionally, we excluded subjects who were diagnosed with malignant neoplasm (C00-C97) or in situ neoplasm (D00-04, D09) based on the 10th edition of the International Classification of Diseases [ICD-10] between 2002 and 2004 (n = 35,136), had a history of cancer in a self-reported questionnaire between 2002 and 2004 (n = 1218), died between 2002 and 2004 (n = 1401), taken insulin for more than 90 days between 2002 and 2003 (n = 66), newly diagnosed with diabetes between 2004 and 2015 (n = 69,985), prescribed metformin before being diagnosed with diabetes between 2002 and 2003 (n = 1028), prescribed metformin without a diabetes diagnosis between 2002 and 2015 (n = 2667), prescribed metformin for less than 90 days between 2002 and 2003, but prescribed metformin for more than 90 days between 2002 and 2015 (n = 21,167), participated in the study for 30 days or less (n = 147), or had any missing values for confounder variables (n = 20,030). The above exclusion conditions were not mutually exclusive. Applying all the exclusion conditions, 191,364 of the participants were excluded. Overall, 323,430 were included in the final analysis.
To examine the effect of metformin in patients with diabetes, the subjects were divided into three groups according to the diagnosis of DM and metformin usage. Metformin users were individuals with DM who had been prescribed metformin for more than 90 days between 2002 and 2003. Metformin non-users were individuals with DM who had been prescribed metformin for less than 90 days or never been prescribed it during the entire study. The no DM group consisted of individuals who had not been diagnosed with DM and had never used anti-diabetic medication. If subjects were prescribed metformin for less than 90 days during the baseline period (2002–2003) but had taken metformin for more than 90 days during the entire study period, these cases were excluded as they would not fall into any of the three groups (Fig. 1). In addition, even if metformin was prescribed for indications other than DM, individuals who meet this condition were also excluded because they did not meet the criteria for metformin users or metformin non-users (Fig. 1).
This study was approved by the Institutional Review Board of Chungbuk National University (CBNUH-2019-12-001) and followed the guidelines of the Declaration of Helsinki (1975).
The operational definitions of DM and CRC
We defined an onset of DM if any of the following conditions were satisfied: (1) the participants had diabetes (ICD-10 code: E11-E14) and had been prescribed diabetes-related medication (insulin, sulfonylurea, metformin, thiazolidinedione, dipeptidyl peptidase-4 inhibitor, α-glucosidase inhibitor, sodium-glucose cotransporter-2 inhibitor, glucagon-like peptide (GLP)-1 agonist, and other anti-diabetic drugs), or (2) had a fasting blood glucose (FBG) of 126 mg/dL or higher.
In this study, the incidence of CRC was defined by using only the main ICD code for CRC (ICD-10 code: C18.0-C20.0) at the time of admission during 2005–2015 to reduce the possibility of false positive diagnoses. To further investigate the association between metformin and site-specific risk for CRC (Supplementary Table 1), we classified CRC into three groups according to the specific anatomical site: proximal (ICD-10 code: C18.0-C18.5), distal (ICD-10 code: C18.6-C18.7), and rectum (ICD-10 code: C19.0-C20.0). Subjects with cancer of multiple sites were assigned based on their first CRC diagnosis (Supplementary Table 1).
For the study period, the study start date for metformin users and non-users was defined as the date when they were initially diagnosed with DM. In the no DM group, the first medical examination was defined as the start date. The study end date was defined as the date when CRC was first diagnosed. If CRC did not develop, the end date was established using the following: when the last health screening was done, the last outpatient visit date, the last date of metformin intake, or date of death.
Potential confounders
In this study, we consider confounding variables to control the risk factors associated with CRC. Variables were extracted from the health screening records between 2002 and 2003. We considered age, body mass index (BMI), systolic blood pressure (SBP), glucose, total cholesterol (TC), alanine aminotransferase (ALT), history of hypertension, smoking status, drinking status, physical activity, and income status as confounding variables. History of hypertension, smoking status, alcohol intake, and physical activity were based on a self-reported questionnaire. A history of hypertension was answered “yes” or “no.” Smoking status was divided into those that had never smoked and those that ever smoked. Drinking status was categorized into the following groups: rarely drink (rare), drink less than twice a week (sometimes), and drink more than three times a week (often). Physical activity was classified into three groups: never, sometimes (exercise between one and four times a week), and regular (exercise at least five times per week). Income status was divided into three groups according to their monthly household income: low (≤ 30th percentile), middle (> 30th to ≤ 70th percentile), and high (> 70th percentile).
Statistical analysis
Data are presented as the mean ± standard error for continuous variables and the number and percentage of participants for categorical variables. For group comparisons, an analysis of variance (ANOVA) and chi-squared test were used. The Kaplan-Meier estimator was used to determine whether metformin use affects the development of CRC in people with diabetes. Log-rank tests were conducted to compare the incidence among the three groups. The cumulative incidence rate was computed by subtracting the Kaplan-Meier estimates. Cox proportional hazards regression models were used to control the risk factors and then estimate the hazard ratio of cancer incidence. Depending on the number of confounding variables, we considered four levels of Cox proportional hazards models: (1) Model 1, only age; (2) Model 2, age, smoking status, drinking status, and physical activity; (3) Model 3, BMI, SBP, ALT, TC, household income status, and history of hypertension, in addition to variables in Model 2; and (4) Model 4, glucose levels in addition to the variables in Model 3. All p values were two-sided, and the results were considered significant if the p value was less than 0.05. All statistical analyses were performed using SAS enterprise guide version 7.1 (SAS Inc., Cary, NC) and R studio version 3.3.3.
Results
The study follow-up period was from 2002 to 2015 (median follow-up of 12.8 years). During the follow-up period, of the total 323,430 participants, 2341 (1.33%) of the 175,495 males and 1204 (0.81%) of the 147,935 females were newly diagnosed with CRC.
Table 1 shows the baseline characteristics according to metformin usage and DM diagnosis by sex. Metformin users were older than non-users for both males and females. Metformin users had higher BMI, glucose, and ALT levels but lower total cholesterol levels. Metformin users tended to have more hypertension. In general, metformin users were more likely to drink less, undertake regular physical activity, and have a higher income level.
Figure 2 demonstrates the significant difference for CRC cumulative incidence according to metformin usage and DM diagnosis based on a Kaplan-Meier’s survival curve. Cumulative incidence was highest in metformin non-users in both sexes (males p value < 0.001, females p value = 0.012). At the end of the follow-up period, the cumulative incidence of CRC in metformin non-users, users, and no DM were 2.07%, 1.84%, and 1.28%, respectively, in males and 1.21%, 0.91%, and 0.80% in females.
The results from the Cox proportional hazards regression models are provided in Table 2. Compared with metformin non-users, age-adjusted HRs (95% CIs) of metformin users and the no DM group were 0.64 (0.50–0.83) and 0.65 (0.56–0.75) in males, and 0.59 (0.38–0.93) and 0.84 (0.62–1.13) in females, respectively (Model 1). Model 3 demonstrated that the HRs (95% CIs) of metformin users and the no DM group were 0.66 (0.52–0.86) and 0.69 (0.60–0.80) in males, and 0.58 (0.37–0.92) and 0.87 (0.64–1.17) in females, respectively, after adjusting for smoking status, alcohol consumption, physical activity, BMI, SBP, hypertension history, TC, ALT, and household income status. After further adjusting for glucose levels, the HRs (95% CIs) of metformin users and the no DM group were 0.66 (0.51–0.85) and 0.72 (0.61–0.85) in males, and 0.59 (0.37–0.92) and 0.93 (0.66–1.29) in females, respectively (Model 4).
In Supplementary Table 1, the colorectum was stratified into the proximal colon, distal colon, and rectum according to the anatomical site in a site-specific association. The adjusted HRs (95% CIs) for proximal colon cancer of metformin users and the no DM group were 0.46 (0.25–0.84) and 0.62 (0.43–0.89) in males, respectively, and were not significant for the distal colon and rectal cancer in males. In females, the fully adjusted HRs for all subgroups of the colorectum were not significant.
Discussion
This study retrospectively analyzed the association between metformin use and CRC risk using the NHIS-HEALS data, which is representative of the Korean population. This study showed that metformin usage in diabetic patients is associated with a reduced risk of developing colorectal cancer. This suggests that metformin use has a potential chemopreventive effect against CRC.
The global prevalence of DM in adults has increased in recent decades [18]. Also, its prevalence in Korea was 11.1% in 2013–2015 and is continuously growing [19]. Metformin is the most commonly used drug in patients with DM and is known to have significant benefits for diabetes-related complications. The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends metformin as the first treatment option for managing DM [8].
The underlying mechanism of metformin to lower blood glucose levels is complex and not yet fully understood. It is known to inhibit gluconeogenesis in the liver and enhance insulin sensitivity in peripheral tissue [13]. Besides, it may have pleiotropic actions beyond glycemic control through the activation of AMPK at a molecular level [13, 16]. It has previously been reported that these additional mechanisms of metformin may have a beneficial preventive effect on cancer development [11, 20, 21]. The insulin/insulin-like growth factor (IGF)–1, activated when nutrients are available, contributes to increased cell growth and proliferation [9, 20]. Thus, metformin can reduce plasma insulin levels and has been suggested to indirectly inhibit tumor proliferation through the insulin-lowering effect in individuals with hyperinsulinemia. Another important related pathway involved in cancer growth is the AMPK pathway, which is activated when cells are starved for carbohydrates. At the level of cell signaling, metformin activates AMPK, and inhibits the mammalian target of rapamycin (mTOR) pathway [9]. Since the mTOR signaling pathway is a target for anticancer treatment [22], it is a potential direct mechanism of metformin to prevent and reduce cancer growth through AMPK activation and mTOR inhibition [11].
Several meta-analyses have reported that metformin usage can reduce the overall risk of cancer development by approximately 10 to 40% [23,24,25]. In addition to using metformin, many other confounding factors can affect the development of cancer. Common risk factors for cancer development in DM patients are known to include age, obesity, insufficient physical activity, and history of smoking [21]. The diabetic group who regularly took metformin were more likely to belong to metformin users. Thus, metformin users are more likely to have better health behaviors, such as healthy eating and regular exercise, than diabetic patients who do not take metformin. In addition, metformin users may tend to undergo regular health check-ups. These health behaviors help to lower carcinogenic risks and increase earlier detection of precancerous lesions and adequate treatment, such as colon polypectomy. In this study, metformin users were older and more obese than other groups but undertook regular physical activity, were less likely to smoke, and consumed less alcohol consumption. These compensatory health behaviors can lower the risk of developing cancer. Although variable factors are complex in their carcinogenic effects, metformin can have additional beneficial effects on cancer prevention.
Several agents are known to have a preventive effect on colorectal carcinogenesis. NSAIDs, including aspirin, have been reported in many studies as chemopreventive agents against CRC [4]. While the exact CRC prevention mechanism of NSAIDs has not yet been established, they are known to prevent CRC development by primarily inhibiting COX-2 [26]. The USPSTF provides a Grade B recommendation for initiating low-dose aspirin use for the primary prevention of cardiovascular disease (CVD) and CRC in adults aged 50 to 59 years who have a 10% or greater 10-year CVD risk [7].
Several previous studies have assessed the risk of metformin use and CRC [27,28,29,30]. The findings of previous studies on the association between CRC and metformin usage are not consistent. The reason for different conclusions between the studies is likely to be related to time-related biases [10, 12]. It is widely accepted that, generally, the adenoma-carcinoma sequence explains the carcinogenesis of the colorectum [31]. The normal mucosa of the colorectum proceeds to a malignant tumor through the process of adenomatous polyps. This process requires various genomic mutations, which is estimated to take more than 10 years [32]. Therefore, a sufficient study population and study period are needed to investigate the effect of some agents on the carcinogenesis of the colorectum. A well-designed recent cohort study reported an inverse association between long-term administration of metformin and CRC risk [27]. The present study shows that metformin usage in patients with diabetes has a beneficial preventive effect on CRC risk in comparison to metformin non-users, and the risk reduction is as low as that of non-diabetic patients. Further research and analysis are needed to clarify the potential clinical benefits of metformin.
This study has several limitations to consider during interpretation. Firstly, several potential confounding factors have been adjusted for, but some residual factors could not be completely controlled in this study, such as lifestyle and underlying genetic or familial factors due to the lack of information in the NHIS-HEALS cohort data. Secondly, there is limited information about the risk factors for CRC, such as a history of inflammatory bowel disease and meat consumption, because the NHIS-HEALS cohort did not provide this information. Thirdly, since the NHIS cohort data is not linked with the Korea Central Cancer Registry Data by the National Cancer Center in Korea, the incidence of CRC in this study might be inaccurate, and there is a possibility of mismatching the actual cancer development. For this reason, colorectal cancer in this study was defined using only the primary diagnosis at hospitalization to reduce the likelihood of false positives. Fourthly, since NHIS data has only the prescription records of patients, it was not possible to confirm that the actual metformin users took their medicine as prescribed. Also, since the prescription duration does not precisely match when the medicine is used, we did not consider the duration of metformin usage. We consider these points to be common limitations of retrospective cohort studies using medical records.
Despite these limitations, this study suggests that real-world metformin usage is associated with a reduced risk of developing colorectal cancer over a relatively long duration. Another strength is that the NHIS-HEALS cohort provided by NHIS, representing the entire Korean population, is based on real-world measurements in a clinical setting. Besides, few studies have investigated the relationship between metformin exposure and CRC risk in Korea.
In conclusion, metformin users with diabetes appear to have a significantly lower risk of CRC compared with metformin non-users. This finding suggests that metformin usage could have a potential preventive effect for CRC.
Data availability
This study used NHIS-HEALS cohort data (NHIS-2019-2-008), which were released by the National Health Insurance Service (NHIS). Access to NHIS-HEALS data is available from the website of NHIS (https://nhiss.nhis.or.kr) after completing the application process and receiving approval (http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do).
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut. 66(4):683–691
Jung KW, Won YJ, Kong HJ, Lee ES (2019) Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat 51(2):417–430
Umezawa S, Higurashi T, Komiya Y, Arimoto J, Horita N, Kaneko T, Iwasaki M, Nakagama H, Nakajima A (2019) Chemoprevention of colorectal cancer: past, present, and future. Cancer Sci 110(10):3018–3026
Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, Chaussade S, Baron JA (2009) Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst 101(4):256–266
Rothwell PM, Wilson M, Elwin C-E, Norrving B, Algra A, Warlow CP, Meade TW (2010) Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376(9754):1741–1750
Bibbins-Domingo K (2016) Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 164(12):836–845
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB (2018) Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 41(12):2669–2701
Gonzalez-Angulo AM, Meric-Bernstam F (2010) Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res 16(6):1695–1700
Higurashi T, Nakajima A (2018) Metformin and colorectal cancer. Front Endocrinol (Lausanne) 9:622
Pollak MN (2012) Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2(9):778–790
Suissa S, Azoulay L (2012) Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35(12):2665–2673
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia. 60(9):1577–1585
Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 510(7506):542–546
Mclntyre H, Paterson C, Ma A, Ravenscroft P, Bird D, Cameron D (1991) Metformin increases insulin sensitivity and basal glucose clearance in type 2 (non-insulin dependent) diabetes mellitus. Aust NZ J Med 21(5):714–719
Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F (2012) Cellular and molecular mechanisms of metformin: an overview. Clin Sci 122(6):253–270
Seong SC, Kim Y-Y, Park SK, Khang YH, Kim HC, Park JH, Kang HJ, Do CH, Song JS, Lee EJ, Ha S, Shin SA, Jeong SL (2017) Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open 7(9):e016640
Ogurtsova K, da Rocha FJ, Huang Y, Linnenkamp U, Guariguata L, Cho NH et al (2017) IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 128:40–50
Lee J, Kang HT, Lim HJ, Park B (2018) Trends in diabetes prevalence among Korean adults based on Korean National Health and Nutrition Examination Surveys III–VI. Diabetes Res Clin Pract 138:57–65
Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG (2017) Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 60(9):1639–1647
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D (2010) Diabetes and cancer: a consensus report. CA Cancer J Clin 60(4):207–221
Meric-Bernstam F, Gonzalez-Angulo AM (2009) Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27(13):2278–2287
Zhang P, Li H, Tan X, Chen L, Wang S (2013) Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 37(3):207–218
Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, la Vecchia C, Mancia G, Corrao G (2012) Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist 17(6):813–822
Noto H, Goto A, Tsujimoto T, Noda M (2012) Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 7(3)
Grossman HB (2003) Selective COX-2 inhibitors as chemopreventive and therapeutic agents. Drugs Today (Barcelona, Spain: 1998) 39(3):203–212
Bradley MC, Ferrara A, Achacoso N, Ehrlich SF, Quesenberry CP Jr, Habel LA (2018) A cohort study of metformin and colorectal cancer risk among patients with diabetes mellitus. Cancer Epidemiol Biomark Prev 27(5):525–530 Epub 2018/05/03
Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE (2011) Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care 34(10):2323–2328
Bodmer M, Becker C, Meier C, Jick SS, Meier CR (2012) Use of metformin is not associated with a decreased risk of colorectal cancer: a case–control analysis. Cancer Epidemiol Prev Biomark 21(2):280–286
Kowall B, Stang A, Rathmann W, Kostev K (2015) No reduced risk of overall, colorectal, lung, breast, and prostate cancer with metformin therapy in diabetic patients: database analyses from Germany and the UK. Pharmacoepidemiol Drug Saf 24(8):865–874
Morson B (1974) The polyp-cancer sequence in the large bowel. SAGE Publications
Leslie A, Carey FA, Pratt NR, Steele RJ (2002) The colorectal adenoma-carcinoma sequence. Br J Surg 89(7):845–860
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
HT Kang and J Kim contributed to concept formation and design of the study protocol and interpretation of data. JW Lee designed the study, managed data and drafted the manuscript. EU Choi managed data, suggested the analytical strategy, performed data analysis, and drafted the article. YS Kim and Y Kim contributed to concept formation and project administration. YE Han, HS Kim and YJ Bae contributed to data curation, methodology and validation. All other authors contributed to the study design and data acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Institutional Review Board of Chungbuk National University (CBNUH-2019-12-001).
Consent to participate
Informed consent was waived because data analyses were performed retrospectively with anonymous data derived from the South Korean National Health Insurance Service database.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Lee, Jw., Choi, EA., Kim, YS. et al. Metformin usage and the risk of colorectal cancer: a national cohort study. Int J Colorectal Dis 36, 303–310 (2021). https://doi.org/10.1007/s00384-020-03765-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03765-x