Abstract
Background
Colorectal anastomoses in patients with colorectal cancer carry a high risk of leakage. Indocyanine green fluorescence angiography (ICG-FA) is a new technique that allows surgeons to assess the blood perfusion of the anastomosis during operation. This meta-analysis aimed to evaluate whether ICG-FA could prevent anastomotic leakage (AL) in colorectal surgery.
Methods
Four databases (PubMed, Embase, Web of Science, and Cochrane Library) were searched to identify suitable literatures until March 2020 that compared AL rates between intraoperative use and non-use of ICG-FA in colorectal surgery for cancer. The Review Manager 5.3 software was used to perform the statistical analysis. Evaluation of articles quality and analysis for publication bias were also conducted.
Results
Thirteen studies of 4037 patients were included in the meta-analysis. The study included 1806 patients in the ICG group and 2231 patients in the control group. The pooled incidence of AL in ICG group was 3.8% compared with 7.8% in control group. There was a significant difference in AL rate with or without use of ICG-FA (OR 0.44; 95% CI 0.33–0.59; P < 0.00001). Reoperation rates were 2.6% and 6.9% in ICG and control groups, respectively. Application of intraoperative ICG-FA was associated with a lower risk of reoperation (OR 0.39; 95% CI 0.16–0.94; P = 0.04). Overall complication rate was 15.6% in the ICG group compared with 21.2% in the control group. Overall complications were significantly reduced when using ICG-FA (OR 0.62; 95% CI 0.47–0.82; P = 0.0008). Mortality rate was not statistically different with or without the use of ICG-FA (OR 1.22; 95% CI 0.20–7.30; P = 0.83).
Conclusion
The results revealed that ICG-FA reduced risks of AL, reoperation, and overall complications for colorectal cancer patients undergoing colorectal surgery. Well-designed RCTs are needed to confirm the usefulness of intraoperative ICG-FA for preventing surgical complications like AL and reoperation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anastomotic leakage (AL) is one of the fatal surgical complications after colorectal resection for patients with cancer. The rates of leakage were reported to be from 2 to 24% [1,2,3,4]. The occurrence of AL leads to prolonged hospitalization, high risk of reoperation, increased local recurrence rate and big chance of permanent stoma, and even shorten survival time [5,6,7]. Despite technical advances, anastomosis is still accompanied by high risk of leakage during colorectal surgery.
Many studies have identified a variety of risk factors for AL. Some are unchangeable such as male sex [8, 9], body mass index [10, 11], ASA score [12], preoperative chemotherapy [13], and tumor diameter [14], while other factors including anastomotic tension and poor anastomotic blood supply can be avoidable. Although it is difficult to determine which factors are critical for incidence of AL, adequate blood perfusion has been well-recognized as one of the key elements for preventing AL in colorectal surgery for cancer [3, 15].
Indocyanine green fluorescence angiography has been widely used in many surgical fields [16,17,18], including gastrointestinal surgery. ICG-FA allows the surgeon to visualize the blood supply and avoid insufficient perfusion of the anastomosis in colorectal surgery. The diluted indocyanine green is injected into the vein system, and the signal is observed by the fluorescence laparoscopy system. When indocyanine green entered the observation area, fluorescence is visualized and blood supply is dynamically observed for colorectal anastomosis. Four meta-analyses [19,20,21,22] have reported that ICG-FA is useful in preventing AL in colorectal surgery, but most of them were not convincing because the number of included studies and patients were limited and small. Lately, some high-quality studies [23,24,25,26,27,28] have been published and reported promising results regarding ICG-FA in prevention of AL after colorectal surgery for cancer. Therefore, this updated meta-analysis was performed to show whether this technology could decrease the surgical morbidity for patients with colorectal cancer. The postoperative complications, in particular, AL rates, were the main interest of this study.
Materials and methods
Search strategy
Databases including PubMed, Embase, Web of Science, and Cochrane Library were searched to identify suitable literature until March 2020 comparing AL rates in colorectal surgery between intraoperative use and non-use of ICG-FA. A combination of medical subject heading (Mesh) terms and text words were used. And we searched with the following Mesh words: “indocyanine green” [Mesh], “rectal neoplasms” [Mesh], “colorectal neoplasms” [Mesh], “anastomotic leakage” [Mesh]. The following text words were used: “ICG,” “rectal cancer,” “rectum neoplasms,” “neoplasm, rectum,” “rectal tumor,” “neoplasms, rectal,” “cancer of rectum,” “rectum cancer,” “colorectal carcinoma,” “colorectal cancer,” “colorectal tumor,” “anastomotic leak,” and “anastomotic dehiscence” using the “OR” for each concept. Each concept was combined with “AND”. The Mesh terms and text words are shown in Table 1. No search limits were applied, and all languages were included. The reference lists of all relevant articles were screened to identify other potential articles.
Study selection and selection criteria
Studies were selected if (1) the patients were diagnosed as primary colorectal cancer by endoscopic and preoperative pathological examination, and underwent colorectal resection with anastomosis and (2) the study included two groups: ICG-FA group and control group. Studies were excluded if (1) study contained patients who received Hartmann’s procedure, Miles ’ procedure, or transanal endoscopic microsurgery; (2) the surgical outcomes were not reported in detail; (3) it is a single study without control group; and (4) they are data missing studies, review articles, letters, case reports, and meta-analyses.
The identified studies were screened by DL Liu and L Liu independently, according to the above criteria. First, studies were screened by titles and abstract. And then the full texts of the remaining studies were examined to decide whether they were suitable for inclusion. Disagreements on the eligibility of a study would result in the assessment of these studies by two additional reviewers, ZQ Zhu and LC Liang, to reach a consensus. The publication types such as review articles, letters, case reports, meta-analyses, and studies lacking necessary data were also excluded.
Data extraction
From each study, the following descriptive information was extracted: publication year, country where the study was conducted, study design, type of operation, cancer type, tumor distance from the anal verge, ICG dose, ICG imaging system, number of patients, basic information of patients, and the total number of AL and AL rate. DLL and LCL independently extracted all these available data. We defined the primary outcome as the rate of AL and the secondary outcome was re-operation rate.
Assessment of the quality
Qualities of the selected studies were assessed according to the Newcastle-Ottawa scale. Four domains were covered including the quality of patient selection, ascertainment of exposure, comparability of groups, and outcomes of patients. The total NOS score ranges from 0 to 9, and a score of ≥ 6 indicates high quality. Quality assessment was executed by two authors, and any disagreement was resolved through discussion.
Statistical analysis
Meta-analysis was performed using the RevMan 5.3 software provided by Cochrane Collaboration Network. The odds ratios (OR) with 95% confidence intervals were used to calculate the total effect. Heterogeneity among the studies was assessed using χ2 and I2 statistics. When there is statistical heterogeneity between pooled studies (I2 > 40%), the random effect model should be used; otherwise, a fixed effect model should be used for analysis. Statistical significance was at P ≤ 0.05. Subgroup and sensitivity analyses were conducted to explore heterogeneity, and publication bias was assessed using funnel plots, if necessary.
Results
Study selection
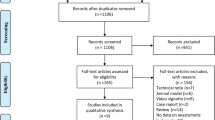
A total of 248 potentially eligible studies were identified and reviewed. After the exclusion of duplicates, 195 publications remained. Then 137 studies were excluded through reviewing title or abstract because they did not meet the inclusion criteria. Of the remaining 58 articles, 45 studies were excluded after full-text review. Finally, thirteen studies were included [23,24,25,26,27,28,29,30,31,32,33,34,35]. The process of systematic literature searching and eligible study selection is shown in Fig. 1.
Study characteristics
Thirteen studies included a total of 4037 patients in this study. The patients of four studies were Japanese, three were American, two studies were Korean, and other four studies were conducted in Italy, Czech Republic, Germany, and Russia, respectively. Ten of the included studies were retrospective, while three were prospective studies. We summarized the characteristics of each eligible study in Table 2, including publication year, country where study was conducted, study design, type of operation, ICG dose, the number of patients, basic information of patients, and the total number of AL and AL rate. The cancer type and tumor distance from the anal verge of the studies are summarized in the Supplementary Table 2.
Outcome assessment
This meta-analysis included 1806 patients in the ICG group and 2231 patients in the control group. Overall AL rate was 6.0%. The pooled incidence of AL in the ICG group was 3.8% compared with 7.8% in the control group. Results of combined analysis indicated that the use of intraoperative ICG-FA contributed to the decreased risk of AL after colorectal surgery (OR 0.44; 95% CI 0.33–0.59; P < 0.00001; Fig. 2). Then, we conducted subgroup analysis based on retrospective or prospective studies. Both ICG groups had significant lower AL rate compared with the control group’s incidence of AL (OR 0.44; 95% CI 0.31–0.63; P < 0.00001; OR 0.48; 95% CI 0.30–0.77; P = 0.002; Fig. 2). Particularly, among the eight studies concerned with rectal cancer, ICG group had a lower AL rate compared with the control group (OR 0.31; 95% CI 0.19–0.49; P < 0.00001; Fig. 3). Four studies [27,28,29,30] were included for analysis of reoperation risk. Figure 4 shows that the reoperation rates were 2.6% and 6.9% in the ICG and control groups, respectively. Result clearly showed that application of intraoperative ICG-FA was associated with lower reoperation rate after colorectal surgery (OR 0.39; 95% CI 0.16–0.94; P = 0.04; Fig. 4). Six studies [25,26,27, 31, 32, 35] reported overall complications after colorectal surgery. The rates were 15.6% in the ICG group compared with 21.2% in the control group. Overall complications were significantly reduced when using ICG-FA during colorectal surgery (OR 0.62; 95% CI 0.47–0.82; P = 0.0008; Fig. 4). In contrast, mortality rate was not statistically different with or without using ICG-FA during colorectal surgery (OR 1.22; 95% CI 0.20–7.30; P = 0.83; Fig. 4).
Quality/publication bias assessment
Quality assessment of the included studies is presented in Table 3. All studies were judged to be of high quality. A funnel plot (Fig. 5) was generated to assess publication bias. We found that the pooled studies were nearly symmetrical and there was no evidence of significant bias in this meta-analysis.
Discussion
Indocyanine green was firstly reported as a tool to assess hepatic function in the late 1950s [36]. Until now, ICG-FA has been used in colorectal surgery, and brings huge benefit to observe anastomotic blood supply. From the published research, the application of ICG-FA in surgery for colorectal cancer is still in the primary stage [20], and there are no standards and specifications for the dose, injection site, and observation time of ICG. Among the 13 included studies, the intravenous dose of ICG is greatly different. The doses adopted in the included studies were 0.2 mg/kg and 0.25 mg/kg, respectively, or varied from 5 to 10 mg. Dinallo et al. [23] administered a bolus of 2 ml of ICG, while Kin et al. [33] injected 3 ml of ICG before creating the anastomosis. And in the study of Mizrahi et al. [35], 3.5 ml of ICG followed by a 10-ml flush of sterile NS which was injected into a peripheral vein. Moreover, the time to perform ICG-FA is also controversial. In the most included studies, ICG fluorescence was intravenously injected immediately before completion of colorectal mobilization and anastomosis. However, Kim [31] and Skrovina et al. [26] used ICG fluorescence after anastomosis formation.
To date, a variety of techniques have been developed to evaluate intestinal blood perfusion, such as tissue oxygen tension, oxygen spectroscopy, and laser Doppler flowmeter. However, these technologies could not be widely clinically used because of the price of equipment, technical complexity, and inconsistent results [37, 38]. In particular, Doppler ultrasound provides limited information [39] and can even produce inaccurate Doppler signals from the pulsations in the occluded vessels [40]. Due to clinically application of fluorescent laparoscopy, indocyanine green is recognized as a potential tool to real-time monitor blood supply of colorectal anastomosis and reduce risk of AL for patients with colorectal cancer. However, ICG-FA has certain limitations. The assessment may be influenced by several patient-related factors, including blood pressure, and body mass index, and also influenced by ICG technique–related factors including dosage and observation time. In addition, fluorescence intensity of ICG-FA was judged by surgeons subjectively in many published studies. The results were not reliable because the signal might be affected by the characteristics and reliability of the camera system and video shooting conditions. None of laparoscopic ICG fluorescence system could be used to quantify fluorescence signals until now. Some researchers tried to quantify the intensity of ICG fluorescence and correlate it with the adequate blood perfusion of colorectal anastomosis after surgery [41, 42].
First, this meta-analysis showed that intraoperative use of ICG-FA was an effective approach to visualize blood supply and, hence, decreased the risk of AL in colorectal resection. The overall AL rate was 6.0%; the intraoperative injection of ICG-FA decreased AL rate by 4% (from 7.8% for control group to 3.8% for ICG-FA group) after colorectal surgery (OR 0.44; 95% CI 0.33–0.59; P < 0.00001; Fig. 2). The result was in line with published meta-analyses [20, 43], which also reported that ICG-FA contributed to decreased risk of AL after colorectal resection for patients with colorectal cancer. Among the included studies, five studies [24, 25, 28, 31, 32] showed that the AL rate was significantly lower in the ICG group than that in the control group. However, other seven studies [23, 26, 27, 29, 30, 34, 35] reported that AL rates were not statistically different between patients with use or non-use of ICG-FA. We noticed that sample sizes of these seven studies were relatively small. Alekseev et al. [32] reported that ICG-FA did not decrease AL rate of high (at 9–15 cm from anal verge) anastomoses, while a decrease in AL rate was observed for low (4–8 cm) anastomoses. The authors concluded that significant reduced risk of AL was found in patients undergoing low rectal anastomoses. Kin et al. [33] was the only one that found there was no reduction in the incidence of AL when using ICG-FA in colorectal surgery because of selection bias and small sample size.
In order to obtain more reliable results, included studies were divided into two groups, prospective or retrospective studies, for subgroup analysis. Unsurprisingly, ICG-FA contributed to decreased risk of AL when compared with control group upon both prospective and retrospective studies (Fig. 2), which indicated the reliability of our study. In addition, subgroup analysis was conducted to precisely assess risk of AL for rectal cancer. The result showed that the use of intraoperative ICG-FA was also associated with significantly lower incidence of AL in rectal cancer surgery (OR 0.31; 95% CI 0.19–0.49; P < 0.00001; Fig. 3). Due to few studies available, we did not conduct subgroup analysis for colonic resection; therefore, high-quality studies were required in the future. Furthermore, the authors of several studies have commercial associations that might influence the work (Supplementary Table 1). To minimize potential bias, studies were included only if the authors declared that they had no conflict of interest. Among these eight studies, AL risk was significantly reduced when using ICG-FA (OR 0.60; 95% CI 0.43–0.82; P = 0.001; Supplementary Figure 1).
Second, the effectiveness of ICG-FA is reflected by the high frequency of modifications or changes of the original surgical plan. Clinical judgment is still considered to be the most important element for surgeons to avoid colorectal AL. Usually, intestinal perfusion is only roughly estimated by the surgeons, using indicators such as mesenteric tissue color or palpable pulsation. However, inadequate tactile and direct visual feedback in laparoscopic surgery may affect the surgeon’s judgment. Dinallo et al. [23] found that more significant alterations were made to the planned anastomotic site in the ICG-FA group and using ICG-FA led to a change in the surgical plan in 5.6%. And Kudszus et al. [34] reported a change in 14% of patients by ICG-FA. In a recent systematic review, Blanco-Colino et al. [44] report a correction rate of 7.4% for the planned resection line in 555 patients who underwent colorectal surgery with intraoperative fluorescence angiography using ICG. These findings were proved by Jafari et al. [30] who reported a change in 19% of cases in the proximal margin of resection, leading to reduce of AL rate by more than a half overall (60–65%). The use of ICG-FA allows for intraoperative evaluation of bowel perfusion and was used to guarantee a reliable anastomosis.
Third, our results suggested that intraoperative use of ICG-FA could potentially decrease risk of complications and improve the surgical outcomes of colorectal cancer surgery. Figure 4 shows that the reoperation rates were 2.6% and 6.9% in the ICG group and the control group, respectively. It was found that the application of intraoperative ICG-FA was associated with lower reoperation rate after AR for rectal cancer (OR 0.39; 95% CI 0.16–0.94; P = 0.04; Fig. 4). A meta-analysis by Shen [22] reported that the reoperation rates were 0.74% and 4.80% in the ICG and control groups, and the work they did was consistent with our results. Six studies reported overall complications after colorectal surgery. The rate was 15.6% in the ICG group compared with 21.2% in the control group, with statistical significance (OR 0.62; 95% CI 0.47–0.82; P = 0.0008; Fig. 4). Seven studies reported the mortality rate, and there were four deaths in two studies [23, 35]. All deaths occurred in the control group, and mortality rate was not statistically different with or without the use of ICG-FA (OR 1.22; 95% CI 0.20–7.30; P = 0.83; Fig. 4). Given the evidence mentioned above, ICG-FA seems to be a valuable method for prevention of colorectal AL and overall complications in colorectal resection for patients with colorectal cancer.
However, there were some limitations for this meta-analysis. First, one of the limitations was the lack of RCTs. Thirteen studies published until March 2020 were included in this review, and ten of them were retrospective studies while three were prospective studies. High-quality RCTs are needed to verify the results of this meta-analysis. Second, the administered dosage of intraoperative ICG was different, and whether the different doses would influence the findings needs to be illustrated. Third, long-term outcomes were not observed in all included studies. Future studies are required to extend the follow-up time and to explore the impact of ICG-FA on long-term prognosis.
Conclusion
In summary, based on this study, we concluded that ICG-FA could lower the risk of AL rate and decrease the probability of reoperation in patients after colorectal resection for cancer. Follow-up studies and well-designed RCTs were needed to confirm the usefulness of intraoperative ICG-FA for preventing surgical morbidity.
References
Ellebaek MB, Rahr HB, Boye S, Fristrup C, Qvist N (2019) Detection of early anastomotic leakage by intraperitoneal microdialysis after low anterior resection for rectal cancer: a prospective cohort study. Color Dis 21(12):1387–1396
Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H (2013) Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg 257(4):665–671
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208(2):269–278
Sverrisson I, Folkvaljon F, Chabok A, Stattin P, Smedh K, Nikberg M (2019) Anastomotic leakage after anterior resection in patients with rectal cancer previously irradiated for prostate cancer. Eur J Surg Oncol 45(3):341–346
Ptok H, Marusch F, Meyer F, Schubert D, Gastinger I, Lippert H, Study Group Colon/Rectum Carcinoma (Primary Tumour) (2007) Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg 94(12):1548–1554
Merkel S, Wang WY, Schmidt O et al (2001) Locoregional recurrence in patients with anastomotic leakage after anterior resection for rectal carcinoma. Color Dis 3(3):154–160
Ha GW, Kim JH, Lee MR (2017) Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Oncol 24(11):3289–3299
Peeters KC, Tollenaar RA, Marijnen CA et al (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 92(2):211–216
Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R (2004) Risk factors for anastomotic leakage after anterior resection of the rectum. Color Dis 6(6):462–469
Yamamoto S, Fujita S, Akasu T, Inada R, Moriya Y, Yamamoto S (2012) Risk factors for anastomotic leakage after laparoscopic surgery for rectal cancer using a stapling technique. Surg Laparosc Endosc Percutan Tech 22(3):239–243
Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio VW (2003) Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg 7(4):558–561
Brandl A, Czipin S, Mittermair R, Weiss S, Pratschke J, Kafka-Ritsch R (2016) Transanal drainage tube reduces rate and severity of anastomotic leakage in patients with colorectal anastomosis: a case controlled study. Ann Med Surg 6:12–16
Qu H, Liu Y, Bi DS (2015) Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 29(12):3608–3617
Rodriguez-Ramirez SE, Uribe A, Ruiz-Garcia EB, Labastida S, Luna-Perez P (2006) Risk factors for anastomotic leakage after preoperative chemoradiation therapy and low anterior resection with total mesorectal excision for locally advanced rectal cancer. Rev Investig Clin 58(3):204–210
Thompson SK, Chang EY, Jobe BA (2006) Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery 26(3):131–136
Kusano M, Tajima Y, Yamazaki K, Kato M, Watanabe M, Miwa M (2008) Sentinel node mapping guided by indocyanine green fluorescence imaging: a new method for sentinel node navigation surgery in gastrointestinal cancer. Dig Surg 25(2):103–108
Lin J, Lin LS, Chen DR, Lin KJ, Wang YF, Chang YJ (2020) Indocyanine green fluorescence method for sentinel lymph node biopsy in breast cancer. Asian J Surg
Lu J, Huang CM (2019) Exploration and development of indocyanine green fluorescence applied in laparoscopic splenic hilum lymph node dissection for gastric cancer. Zhonghua Zhong Liu Za Zhi 41(12):900–903
Degett TH, Andersen HS, Gogenur I (2016) Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbeck's Arch Surg 401(6):767–775
Shen R, Zhang Y, Wang T (2018) Indocyanine green fluorescence angiography and the incidence of anastomotic leak after colorectal resection for colorectal cancer: a meta-analysis. Dis Colon Rectum 61(10):1228–1234
Hu MH, Huang RK, Zhao RS, Yang KL, Wang H (2017) Does neoadjuvant therapy increase the incidence of anastomotic leakage after anterior resection for mid and low rectal cancer? A systematic review and meta-analysis. Color Dis 19(1):16–26
Shen Y, Yang T, Yang J, Meng W, Wang Z (2020) Intraoperative indocyanine green fluorescence angiography to prevent anastomotic leak after low anterior resection for rectal cancer: a meta-analysis. ANZ J Surg
Dinallo AM, Kolarsick P, Boyan WP, Protyniak B, James A, Dressner RM, Arvanitis ML (2019) Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am J Surg 218(1):136–139
Hasegawa H, Tsukada Y, Wakabayashi M, Nomura S, Sasaki T, Nishizawa Y, Ikeda K, Akimoto T, Ito M (2020) Impact of intraoperative indocyanine green fluorescence angiography on anastomotic leakage after laparoscopic sphincter-sparing surgery for malignant rectal tumors. Int J Color Dis 35(3):471–480
Ishii M, Hamabe A, Okita K, Nishidate T, Okuya K, Usui A, Akizuki E, Satoyoshi T, Takemasa I (2020) Efficacy of indocyanine green fluorescence angiography in preventing anastomotic leakage after laparoscopic colorectal cancer surgery. Int J Color Dis 35(2):269–275
Skrovina M, Bencurik V, Martinek L et al (2020) The significance of intraoperative fluorescence angiography in miniinvasive low rectal resections. Wideochir Inne Tech Maloinwazyjne 15(1):43–48
Wada T, Kawada K, Hoshino N, Inamoto S, Yoshitomi M, Hida K, Sakai Y (2019) The effects of intraoperative ICG fluorescence angiography in laparoscopic low anterior resection: a propensity score-matched study. Int J Clin Oncol 24(4):394–402
Watanabe J, Ishibe A, Suwa Y, Suwa H, Ota M, Kunisaki C, Endo I (2020) Indocyanine green fluorescence imaging to reduce the risk of anastomotic leakage in laparoscopic low anterior resection for rectal cancer: a propensity score-matched cohort study. Surg Endosc 34(1):202–208
Boni L, Fingerhut A, Marzorati A, Rausei S, Dionigi G, Cassinotti E (2017) Indocyanine green fluorescence angiography during laparoscopic low anterior resection: results of a case-matched study. Surg Endosc 31(4):1836–1840
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27(8):3003–3008
Kim JC, Lee JL, Yoon YS, Alotaibi AM, Kim J (2016) Utility of indocyanine-green fluorescent imaging during robot-assisted sphincter-saving surgery on rectal cancer patients. Int J Med Robot Comput Assist Surg 12(4):710–717
Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I (2020) A study investigating the perfusion of colorectal anastomoses using FLuorescence AnGiography: results of FLAG randomized trial. Color Dis
Kin C, Vo H, Welton L, Welton M (2015) Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis Colon Rectum 58(6):582–587
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck's Arch Surg 395(8):1025–1030
Mizrahi I, Abu-Gazala M, Rickles AS, Fernandez LM, Petrucci A, Wolf J, Sands DR, Wexner SD (2018) Indocyanine green fluorescence angiography during low anterior resection for low rectal cancer: results of a comparative cohort study. Tech Coloproctol 22(7):535–540
Leevy CM, Mendenhall CL, Lesko W, Howard MM (1962) Estimation of hepatic blood flow with indocyanine green. J Clin Invest 41:1169–1179
Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW (2019) Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc 33(5):1640–1649
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc 31(10):4184–4193
Boyle NH, Manifold D, Jordan MH, Mason RC (2000) Intraoperative assessment of colonic perfusion using scanning laser Doppler flowmetry during colonic resection. J Am Coll Surg 191(5):504–510
Nickele C, Nguyen V, Fisher W, Couldwell W, Aboud E, David C, Morcos J, Charalampaki C, Arthur A (2019) A pilot comparison of multispectral fluorescence to indocyanine green videoangiography and other modalities for intraoperative assessment in vascular neurosurgery. Oper Neurosurg 17(1):103–109
Sherwinter DA, Gallagher J, Donkar T (2013) Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Color Dis 15(1):91–96
Chang YK, Foo CC, Yip J, Wei R, Ng KK, Lo O, Choi HK, Law WL (2019) The impact of indocyanine-green fluorescence angiogram on colorectal resection. Surgeon 17(5):270–276
Rausa E, Zappa MA, Kelly ME, Turati L, Russo A, Aiolfi A, Bonitta G, Sgroi LG (2019) A standardized use of intraoperative anastomotic testing in colorectal surgery in the new millennium: is technology taking over? A systematic review and network meta-analysis. Tech Coloproctol 23(7):625–631
Blanco-Colino R, Espin-Basany E (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22(1):15–23
Author information
Authors and Affiliations
Contributions
DLL and ZQZ were involved in the study conception and design. DLL and LCL performed the data acquisition. DLL and LCL performed the analysis and interpretation of the data. DLL and LL drafted the manuscript. LCL and LL critically revised the manuscript. DLL and LL submitted the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Fig. 1
(PNG 1080 kb)
Supplementary Table 1
(DOCX 16 kb)
Supplementary Table 2
(DOCX 17 kb)
ESM 1
(DOC 64 kb)
ESM 2
(DOCX 35 kb)
Rights and permissions
About this article
Cite this article
Liu, D., Liang, L., Liu, L. et al. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int J Colorectal Dis 36, 57–66 (2021). https://doi.org/10.1007/s00384-020-03741-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03741-5