Abstract
Background
Myenteric ganglionitis is a disorder that causes intestinal motor dysfunction. It may be caused due to neoplastic, central nervous system, or systemic infectious disorders. However, some cases are considered to be idiopathic in origin.
Case presentation
A 33-year-old man was admitted to the hospital with sudden severe abdominal pain accompanied by watery diarrhea. Computed tomography imaging revealed edema of the entire small intestinal wall without ischemic changes. Detailed examination could not be performed for identifying the cause of abdominal pain because of the patient’s worsened general condition, and he died 7 days after onset. The autopsy results confirmed the cause of the patient’s severe abdominal pain as an idiopathic myenteric ganglionitis.
Conclusion
Some patients with idiopathic myenteric ganglionitis might die without a definitive diagnosis during their lifetime because of the rarity of this disease. When encountering severe intestinal motility abnormalities of unknown cause, physicians should consider idiopathic myenteric ganglionitis when choosing therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal pseudo-obstruction (IPO) is a clinical entity characterized by intractable intestinal obstruction without any organic abnormalities [1]. Currently, IPO is classified as acute or chronic according to disease duration and as primary, secondary, or idiopathic according to the underlying abnormality [2,3,4]. IPO exhibiting chronic disease progression of unknown cause, termed as chronic idiopathic intestinal pseudo-occlusion (CIIP), can be pathologically classified as myopathic, neuropathic, or mesenchymopathic [5, 6]. An abnormal autoimmune response against intestinal skeletal muscle of the muscularis propria, myenteric ganglionic cells, or Cajal cells is believed to be a major cause of CIIP [7, 8]. However, approximately 25% of CIIP cases show no histopathological changes [9]. Therefore, IPO is considered a rare disease entity because of the difficulty of making a definite diagnosis. In addition, although it is rarely observed, there are a few reports of myenteric ganglionitis as a fatal disorder with an acute/subacute clinical course.
Herein, we describe the case of a young male patient who died due to idiopathic myenteric ganglionitis (IMG) accompanied by sudden abdominal pain. At autopsy, his disorder was clinicopathologically identified as IMG of the small intestine, characterized by severe lymphocytic infiltration and loss of ganglion cells in the small intestinal Auerbach’s myenteric plexus. Currently, only seven cases of clinicopathologically proven IMG have been reported in the literature [8, 10,11,12]. We also review the clinicopathological features of previously reported cases of IMG.
Clinical summary
A 33-year-old man with no previous personal medical history or family history visited a local clinic with primary complaints of fever, abdominal pain, and vomiting. Computed tomography (CT) imaging demonstrated thickening of the small intestinal wall. However, as his blood test disclosed a normal inflammatory response, he was followed up on an outpatient basis. After 3 days of onset, he developed severe abdominal pain accompanied by watery diarrhea, and he was hospitalized 4 days after the onset. At day 5 after onset, his blood test revealed a high inflammatory response and a decreased number of platelets. Contrast-enhanced CT imaging showed edema of the entire small intestinal wall, but there were no ischemic changes. Although we considered the possibility of superior mesenteric artery embolism or inflammatory bowel disease, further examination could not be performed because of the patient’s worsened general condition. He was treated with carbapenem antibiotics, immunoglobulin preparations, and thrombomodulin based on the clinical diagnosis of acute enteritis and disseminated intravascular coagulation (DIC) syndrome. However, 6 days after onset, he developed metabolic acidosis, hyperkalemia, hypocalcemia, and hypoglycemia, and he died on the 7th day.
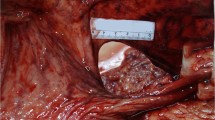
An autopsy of the systemic organs was performed 2 h and 48 min after his death. Macroscopic examination revealed widespread severe submucosal edema and hemorrhage throughout the small intestine and thinning of the intestinal wall of the ileum. The colon also demonstrated thinning of the intestinal wall and mild submucosal hemorrhage (Fig. 1). There was no obvious thrombus formation in any of the arteries supplying the small and large intestines. Mild submucosal hemorrhage was observed in the stomach.
Macroscopic features of the small intestine affected by intestinal myenteric ganglionitis. Almost the entire small intestine (jejunum to ileum) demonstrated dilatation of the lumen and thinning of the wall, in addition to erosion and submucosal hemorrhage (a). However, the ileum revealed dilatation, thinning of the wall, and submucosal hemorrhage, and the colon was relatively well preserved (b)
Histopathologically, changes resembling ischemic enteritis were detected in the mucosal layer of the small intestine (Fig. 2a). The submucosal layer of the small intestine was affected by congestion, edema, and mild chronic inflammatory cell infiltration. The most characteristic feature of the small intestine was localized infiltration of neutrophils, plasma cells, and lymphocytes in the Auerbach’s myenteric plexus and its surrounding muscularis propria. Aggregates of histocytes engulfing nuclear dust were conspicuous in the muscularis propria of the small intestine (Fig. 2b). In the Auerbach’s myenteric plexus, degeneration and loss of ganglionic cells were prominent, with destruction of its surrounding muscularis propria and peripheral nerves of the myenteric plexus (Fig.2c, d). CD117-positive interstitial cells of Cajal were not detectable in the small intestinal lesion. These destructive pathological features were most prominent in the jejunum, followed by the duodenum and then the ileum.
Microscopic features of intestinal myenteric ganglionitis in the small intestine. The small intestinal mucosal layer demonstrated mild ischemic enterocolitis-like changes, and edema affected the submucosal layer (a). The small intestinal muscularis propria layer was severely affected by inflammatory cell infiltration (b). Auerbach’s plexus was destroyed by inflammatory cells, including small- to medium-sized lymphocytes and macrophages, and a few degenerated ganglion cells could be found in the lesion (c). Neurofilament protein immunohistochemistry revealed marked peripheral nerve loss of Auerbach’s plexus (d). In addition, prominent infiltration of CD3-positive T cell lymphocytes was observed in the small intestinal muscularis propria layer (e). In the small intestinal muscularis propria layer lesion, CD8-positive T cell lymphocytes (f) infiltrated more frequently than CD4-positive T cell lymphocytes (g). Several CD68-positive macrophages also aggregated in the lesion (h)
Immunohistochemical examination showed that the infiltrating inflammatory cells in the small intestinal destructive lesion were predominantly CD3-positive T cell lymphocytes (Fig. 2e). More of these T cells expressed CD8 than CD4 (Fig. 2f, g). In the background, CD68-positive histiocytes were conspicuous in the lesion (Fig. 2h). A hemorrhagic tendency and/or DIC-associated microthrombus formation were observed in other organs, but no neoplastic infectious lesions were found. The patient’s condition was finally clinicopathologically diagnosed as IMG.
Discussion
This report describes a case of a young male patient who died from IMG within a short time period after onset. The patient exhibited the following clinicopathological and radiological characteristics: acute progressive abdominal pain, vomiting and watery diarrhea, thickened small intestinal wall demonstrating edema, and localized inflammatory cell infiltration with marked destruction of the myenteric plexus of the small intestinal muscularis propria.
No clinical or pathological lesions were detected in the systemic organs, suggesting the possibility of secondary IPO such as paraneoplastic syndrome related to pulmonary small cell carcinoma and neuroblastoma or infectious diseases [10, 11]. In contrast, localized lymphoplasmacytic infiltration with macrophage accumulation was detected in the small intestinal muscularis propria layer, and loss of ganglionic cells of the Auerbach’s myenteric plexus was prominent. However, we could not identify the cause of these inflammatory cell infiltrations. Therefore, we believe that the present case was not primary or secondary IPO, but idiopathic IPO, termed as IMG [8, 10,11,12].
IMG is a very rare disorder characterized by inflammatory cell infiltration confined to the myenteric plexus of the intestinal tract in young adults, which causes severe intestinal motility disorders [8, 10]. Until date, seven IMG cases have been reported, including our case; the clinicopathological findings of these cases are summarized in Tables 1 and 2 [8, 10,11,12]. Of these seven cases, four were males and three were females. The average age at onset was 31 years (median, 37.5 ± 17.5 years; range, 20–55 years). The IMG lesions primarily involved the small intestine in three cases and the large intestine in four cases. However, the small intestine was also affected by IMG lesions in two of the four cases primarily involving the large intestine; therefore, it could be assumed that the small intestine tends to be affected by the IMG lesion. Two of the seven patients were tested for anti-Hu antibodies, and both were found to be immunopositive, although no findings were detected suggestive of paraneoplastic syndrome [8, 12]. Anti-Hu antibody is one of the antineuronal autoantibodies associated with paraneoplastic syndrome [13], and it has been suggested that the presence of anti-Hu antibodies leads to the death or apoptosis of myenteric neurons, resulting in gastrointestinal dysfunction and movement disorders [13]. However, at present, it is unclear whether the presence of anti-Hu antibodies is directly associated with nerve damage or is simply a result of nerve damage [12]. Further clinicopathological studies including autoantibodies are required to understand the cause of IMG.
Histopathologically, IMG is characterized by dense lymphoplasmacytic infiltration restricted to the myenteric plexus and by degeneration or loss of myenteric ganglion cells [8]. De Giorgio et al. found that the degree of inflammation and neuronal damage in IMG lesions differed according to the stage of IMG [8]. In the early stages of IMG, the lesions were affected by severe inflammation with degeneration of ganglion cells, but without apparent neuronal loss. On the other hand, there was an evident loss of myenteric ganglion cells with mild inflammatory cell infiltration in the late stages [8]. Although our case was characterized by prominent inflammation with obvious degeneration and loss of myenteric ganglion cells in the IMG lesions, we believe that these histopathological features suggest the transition of disease stage from early to late.
Selective T cell infiltration into the myenteric plexus was the common histopathological finding in six cases of pathologically proven IMG [8, 10,11,12]. De Giorgio et al. suggested that T cell infiltration in IMG contributes to the degeneration and/or loss of the enteric Auerbach’s plexus, based on the immune response against myenteric ganglion cells by both CD4-positive helper T cells and CD8-positive T cytotoxic/suppressor T cells, and that the degeneration and/or loss of myenteric ganglion cells could be due to cytotoxic activity against these cells [11]. In fact, severe T cell infiltration was detected in the lesions of pathologically proven IMG [8, 10,11,12]. In addition, a high expression of chemokine macrophage inflammatory protein-1 alpha (MIP-1 alpha), produced by macrophages, was reported in IMG lesions [14]. It has been reported that MIP-1 alpha is the chemokine that recruits inflammatory mononuclear cells into a lesion [14, 15]. Karpus et al. investigated T cell-mediated autoimmune disease and reported that the production of MIP-1 alpha correlated with increasing disease severity [15]. Although previous studies did not describe macrophage infiltration in IMG lesions, not only T cells but also macrophages were easily detectable in the IMG lesions of the present case. With regard to myenteric ganglion cells, previous reports have described a decreased expression of bcl-2 gene products, which function in apoptosis, in the myenteric ganglion cells of IMG lesions [14, 16]. Unfortunately, we could not determine bcl-2 expression in the myenteric ganglion cells in the IMG lesions, because only a few residual myenteric ganglion cells were observed in the present case. However, these pathological findings appear to support the hypothesis of De Giorgio et al., i.e., T cell infiltration might reflect the immune response directly against the myenteric plexus [11].
In the present case, the infiltration of CD8-positive T cells was predominant. The CD4/CD8 ratios of infiltrative T cells in IMG lesions have been reported in three pathologically proven IMG cases [8, 10,11,12]. In two cases, neither CD4-positive nor CD8-positive T cells were predominant [8, 11]. In the remaining one case, reported by Racalbuto et al., CD4-positive T cells were predominant [10]. The timing of pathological examinations of the IMG lesions was different between the cases; therefore, we suggest that the differences in the CD4/CD8 ratio in IMG lesions depend on the disease stage or disease activity.
Among the seven patients with pathologically proven IMG, two died due to the disorder, including one patient with colonic perforation. However, the other patient who died had no perforation of the alimentary tract, and there appeared to be no correlation between perforation of the alimentary tract and prognosis. However, histological observations demonstrated that both these patients had thinned intestinal walls in the IMG lesion compared with patients with a better prognosis. Thinned intestinal walls might be one of the possible features indicating a poor prognosis for IMG. As of now, further accumulation of cases and clinicopathological and radiological analyses are required to elucidate the factors associated with the prognosis of IMG.
Conclusion
IMG is rarely encountered in daily clinical practice. However, when intestinal motility disorders of unknown cause are detected in young adults, IMG should be included in the differential diagnosis. Testing for anti-Hu antibodies and performance of intestinal full-thickness biopsy would also be desirable. The application of steroid pulse therapy has been claimed for IMG treatment [8, 12] because IMG has been believed to be associated with an immune response against myenteric ganglion cells. However, there is currently no established treatment for IMG. Therefore, further accumulation of cases and clinicopathological and radiological analyses are necessary to establish the appropriate treatment for IMG.
References
Dudley HA, Sinclair IS, McLaren IF et al (1958) Intestinal pseudo-obstruction. JR Coll Surg Esinb 3:206–217
Maldonado JE, Gregg JA, Green PA (1970) Chronic idiopathic intestinal pseudoobstruction. Am J Med 49:203–212
Anuras S, Baker CRF (1986) The colon in the pseudoobstructive syndrome. Clin Gastroenterol 15:745–752
Stanghellini V, Corinaldesi R, Barbara L (1988) Pseudo-obstruction syndromes. Baillieres Clin Gastroenterol 2:225–254
De Giorgio R, Camilleri M (2004) Human enteric neuropathies: morphology and molecular pathology. Neurogastroenterol Motil 16:515–531
De Giorgio R, Sarnelli G, Corinaldesi R, Stanghellini V (2004) Advances in our understanding of the pathology of chronic intestinal pseudo-obstruction. Gut 53:1549–1552
Törnblom H, Lang B, Clover L, Knowles CH, Vincent A, Lindberg G (2007) Autoantibodies in patients with gut motility disorders and enteric neuropathy. Scand J Gastroenterol 42:1289–1293
De Giorgio R, Barbara G, Stanghellini V, De Ponti F, Salvioli B, Tonini M et al (2002) Clinical and morphofunctional features of idiopathic myenteric ganglionitis underlying severe intestinal motor dysfunction: a study of three cases. Am J Gastroenterol 97:2454–2459
Stanghellini V, Cogliandro RF, De Giorgio R, Barbara G, Salvioli B, Corinaldesi R (2007) Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil 19:440–452
Racalbuto A, Magro G, Lanteri R, Aliotta I, Santangelo M, Di Cataldo A (2008) Idiopathic myenteric ganglionitis underlying acute ‘dramatic’ intestinal pseudoobstruction: report of an exceptional case. Case Rep Gastroenterol 2:461–468
De Giorgio R, Barbara G, Stanghellini VF, Cogliandro RF, Arrigoni A, Santini D et al (2000) Idiopathic myenteric ganglionitis underlying intractable vomiting in a young adult. Eur J Gastroenterol Hepatol 12:613–616
Basilisco G, Gebbia C, Peracchi M, Velio P, Conte D, Bresolin N, Nobile-Orazio E (2005) Cerebellar degeneration and hearing loss in a patient with idiopathic myenteric ganglionitis. Eur J Gastroenterol Hepatol 17:449–452
Drukker CA, Heij HA, Wijnaendts LC, Verbeke JI, Kaspers GJ (2009) Paraneoplastic gastro-intestinal anti-Hu syndrome in neuroblastoma. Pediatr Blood Cancer 52:396–398
De Giorgio R, Barbara G, Pulsatelli L et al (2000) Chemokine expression and lymphocyte subsets in patients with idiopathic myenteric ganglionitis. Gastroenterology 118:A630 (abstract)
Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD (1995) An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol 155:5003–5010 (abstract)
Warren CFA, Wong-Brown MWW, Bowden NA (2019) BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis 10:177
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izu, A., Homma, T., Hirabayash, M. et al. Idiopathic myenteric ganglionitis as a cause of death in a young male patient with sudden abdominal pain: an autopsy case report. Int J Colorectal Dis 35, 1801–1805 (2020). https://doi.org/10.1007/s00384-020-03631-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03631-w