Abstract
Purpose
To retrospectively review our experience on 84 patients with squamous cell anal canal cancer (SCAC) within 12 months after combined treatment with intensity-modulated RT (IMRT), in terms of acute and early-late toxicity, overall treatment time and interruptions, colostomy-free survival (CFS), and tumor response.
Methods
Acute gastrointestinal (GI), genitourinary (GU), and cutaneous (CU) toxicities were assessed according to Common Toxicity Criteria for Adverse Events (CTCAE) version 4.03. Early-late toxicity was scored using the Radiation Therapy Oncology Group (RTOG) late radiation morbidity scoring system. Tumor response was evaluated with response evaluation criteria in solid tumors (RECIST) v1.1.
Results
Acute toxicity for 84 subjects (100%): severe (≥ G3) GI and skin toxicity was observed in 4 (5%) and 19 patients (23%), respectively. Early-late toxicity for 73 subjects (87%): severe (≥ G3) GI and vulvo-vaginal toxicity was observed in 2 (3%) and 2 (3%) patients, respectively. No acute or early-late severe GU toxicity was reported. A treatment interruption occurred in 65 patients (77%). CFS was 96% (95% CI 89–99) at 6 months and 92% (95% CI 83–96) at 12 months. At 6 months complete response (CR), partial response (PR) and progressive disease (PD) was observed in 70 (83%), 3 (4%), and 7 patients (8%), respectively. At 12 months, CR was observed in 60 patients (81%); eleven patients (15%) experienced PD.
Conclusion
Our study showed an excellent clinical result and very low acute toxicity rates, confirming the IMRT as standard of care for curative treatment of anal cancer patients.
The current trial was registered with the number IEO N87/11
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
Concurrent chemotherapy (ChT) and radiation therapy (RT) has replaced abdominoperineal resection as the primary treatment of invasive squamous cell anal cancer (SCAC) and is the current international standard of care.
The results of a large number of non-randomized and randomized trials confirmed the efficacy and safety of primary chemoradiation [1,2,3,4], which avoids radical surgery with permanent colostomy. However, this attractive strategy confers non-negligible treatment-related implications and long-term side effects, which might be enhanced if higher radiation doses are delivered. The use of innovative RT modalities such as intensity-modulated radiation therapy (IMRT) has led to highly conformal radiation treatments, which allow smaller margins and decreased radiation doses to the normal healthy organs at risk (OARs). The consequent reduction in acute toxicity helps to ensure better compliance and a shorter overall treatment time (OTT), minimizing treatment interruptions, which might compromise the effectiveness of chemoradiation [5,6,7,8,9]. IMRT has also enabled the use of a simultaneous integrated boost (SIB) strategy to treat the macroscopic primary tumor, clinically involved nodal disease and elective target volumes to different total doses. Based on these advantages, IMRT should be considered as the preferred option in treating anal cancer.
The aim of this study is to describe acute and early-late toxicity including compliance and interruptions, within 12 months after combined treatment with IMRT in patients with SCAC at the European Institute of Oncology (IEO), Milan, Italy. Tumor response to the treatment is also reported.
Methods
We retrospectively reviewed the data of 84 patients treated at the Radiotherapy Department of IEO between March 2010 and August 2018. All patients had biopsy-proven/histologically confirmed SCAC and received combined treatment with ChT and IMRT, for which they signed a specific informed consent. In addition, they gave written consent for the use of their anonymous clinical data for research and educational purpose.
The study was part of a research project entitled “Image guided radiotherapy in gastrointestinal malignancies” notified to the Ethical Committee of the IEO, Milan, Italy (No. IEO N87/11), with which all the clinical and technical analyses concerning these modalities, conducted both retrospectively and prospectively, were approved.
Study procedures
All patients underwent a pre-treatment evaluation including physical examination, laboratory data, and proctoscopy with biopsy of anal tumor, endoscopic ultrasound, and chest and abdomino-pelvic computed tomography (CT).
Pelvic magnetic resonance imaging (MRI) was performed whenever possible and 18Fluorodeoxyglucose positron emission tomography/computed tomography (18FDG PET/CT) was requested as second level diagnostic procedure whenever doubts on the stage emerged.
Follow-up was regularly performed by radiation oncologists, alongside medical oncologists. Clinical follow-up was done during and at the end of the RT, every 4 months for the first 2 years and every 6 months up to 5 years, according to clinical conditions. It includes digital rectal examination (DRE), laboratory data, proctoscopy, MRI, and CT or 18FDG PET/CT.
Primary tumor and distant metastases response were evaluated with response evaluation criteria in solid tumors (RECIST) v1.1 [10] during the follow-up period using MRI, proctoscopy, and chest and abdomino-pelvic CT scan 6 months for the first 2 years and every 12 months thereafter.
Progression was classified as loco-regional recurrence or distant failure at any time of observation; loco-regional recurrence is defined as a clinically proven local failure at primary site or in pelvic lymph nodes included in the original RT volume, regardless of any distant failures. Distal failure is defined as the presence of distant metastases outside the RT volume, irrespective of any loco-regional recurrence.
Colostomy-free survival is defined considering both disease-associated and treatment-associated colostomy formation.
Treatment protocol
Radiotherapy
Patients underwent CT simulation and were treated in supine position, with full urinary bladder and empty rectum. A radiopaque marker at the anal verge and in vagina for female patients was placed as reference points for contouring. The gross tumor volume (GTV) was contoured on the CT scan, co-registered with MRI images and with 18FDG PET-CT when available. The GTV included primary tumor and involved lymph nodes. Clinical target volume (CTV) and planning target volume (PTV) were contoured according to Radiation Therapy Oncology Group (RTOG) 0529 and Australasian Gastrointestinal Trials Group (AGITG) guidelines [6, 11]. All patients were treated encompassing the inguinal nodes bilaterally with a prophylactic and/or curative intent.
The elective nodal PTV was planned to receive at least 32 Gy, while the minimum dose was 40 Gy for involved structures PTV and 50 Gy for tumor. The elective low-risk PTV, composed of bilateral external iliac, internal iliac, presacral, and inguinal nodes, received a median dose of 41.4 Gy (range 32.4 Gy–48.6 Gy), and high risk PTV, composed of strictly adjacent tumor tissues volume, received a median dose of 46 Gy (range 40 Gy–56 Gy), while tumor and positive nodes received boost dose up to a total median dose of 56 Gy (range 36Gy–60Gy). Objectives for target volumes were set so that, for PTV, V95 should be at least 95%, V110 ≤ 3%, and ≤ 3% should receive < 93% of prescribed dose.
Dosimetric parameters followed the recommendations of the International Commission on Radiation Units and Measurements (ICRU) reports No. 83 [12].
The boost on macroscopic disease was given using either a sequential boost technique or a SIB strategy.
Some patients received boost with brachytherapy (BRT), according to clinical considerations (performance status, age), tumor stage, and for tumors encompassing no more than one half of the circumference of the anal canal and with no adjacent organ infiltration. Application form was contact BRT intraluminal implantation; a rigid single-channel–shielded cylinder was placed in the anal canal, and an Iridium-192 source was introduced through catheters fitted into the endorectal applicator. Both high-dose rate (HDR) and pulsed-dose rate (PDR) brachytherapies were used.
The prescribed dose was delivered with daily fractions of 1.7–2 Gy, 5 days per week. OARs were also delineated [13]. The dose volume constraints for OARs were adopted from RTOG 0529 and RTOG 0921 trials [6, 14].
Treatment plans were developed using the Varian Eclipse® planning system or Tomotherapy® planning system.
All the patients were treated with IMRT, using either Trilogy® linac or Tomotherapy® or volumetric modulation ARC therapy (VMAT) realized with Trilogy® linac.
All patients underwent an image-guided RT (IGRT).
Chemotherapy
All patients underwent oncologic evaluation to assess concurrent ChT administration.
The regimen administered in 83% of cases (see details in Table 1) consisted in oral fluoropyrimidine plus cisplatin (capecitabine 1650 mg/m2 orally, as 5-day/week regimen concomitant with RT plus cisplatin 60–70 mg/m2 (based on performance and comorbidities) intravenous (i.v.) infusion on day 1 and day 21).
The remaining patients received ChT containing concurrent fluoropyrimidines and mitomycin (capecitabine 1650 mg/m2 orally, as five day/week regimen concomitant with RT or 5-fluorouracil 1000 mg/m2 per day as i.v. continuous infusion days 1–4 and 29–32 plus mitomycin 10 mg/m2 days 1 and 29).
Outcomes
The evaluated outcomes were as follows:
-
Tumor response, evaluated at 6 and 12 months after the end of RT.
-
Incidence of posttreatment colostomy during the first year after the start of RT.
-
Colostomy-free survival (CFS) during the first year after the start of RT.
-
Compliance and overall treatment time (days) and interruptions.
-
Acute toxicity, evaluated from the end of RT to 6 months.
-
Early-late toxicity, evaluated from 6 months after the end of RT to 1 year after the end of RT; patients with progressive disease at 6 months, death in the first 6 months, and lost at follow-up were not considered for this outcome.
Results reporting 3- and 5-year overall survival, progression-free survival, disease-free survival, and late toxicity will be discussed in a subsequent study when mature follow-up will be available.
Toxicity assessment
Acute gastrointestinal (GI), genitourinary (GU), and skin toxicities were assessed according to Common Toxicity Criteria for Adverse Events (CTCAE) version 4.03 [15].
Early-late toxicity (after 6–12 months posttreatment completion) was scored using the RTOG late radiation morbidity scoring system [16]. The maximum toxic effect grade was used for each patient and each event type.
Both any grade and specifically severe (≥ G3) acute and early-late toxicity were individually presented.
Statistical analysis
Exact confidence intervals for binomial proportion were calculated.
The chi-square test (or Fisher’s exact test, when appropriate) and Cochran-Armitage trend test for ordinal variables were used to evaluate the association between patient and treatment characteristics and acute or early-late severe toxicity (i.e., ≥ G3).
The cumulative incidence of colostomy was estimated using the Kalbfleisch and Prentice method [17], considering death as a competing event.
All analyses were performed using SAS software v. 9.4 (SAS Institute, Cary, NC, USA).
A p value less than 0.05 was considered statistically significant.
Results
Patients, tumor, and treatment characteristics are summarized in Table 1.
Pre-RT evaluation was performed with physical examination and laboratory data on all the 84 patients; CT, proctoscopy with anal tumor biopsy, pelvic MRI, and anorectal endoscopic ultrasound were performed in 84 (100%), 84 (100%), 70 (83%), and 48 (57%), respectively. A total of 55 patients (65%) underwent 18FDG PET-CT imaging.
Four patients were defined as M1 due to radiological evidence of distant disease. Metastatic sites were the skin (1), bone and peritoneal (1), and lung and liver (2). None of them were cyto/histologically confirmed, and the treatment plan for pelvic RT was confirmed for obtaining more rapid and effective tumor local control.
Of the entire cohort, 62 patients (74%) were treated with Trilogy® and 22 patients (26%) with Tomotherapy®.
The elective low-risk PTV, composed of bilateral external iliac, internal iliac, presacral, and inguinal nodes, received a median dose of 41.4 Gy (range 32.4–48.6 Gy); high-risk PTV, composed of strictly adjacent tumor tissues volume, received a median dose of 46 Gy (range 40–56 Gy), while tumor and positive nodes received boost dose up to a total median dose of 56 Gy (range 36–60 Gy). The 95% of the prescribed volume received the 95–97% of the prescribed dose. The accepted variation in the dose distribution was 93% < 0.03 cm3 of the PTV < 110%.
BRT was administered in 13 patients (15%) as a boost after 36–50 Gy of external RT: in 3 cases pulsed-dose rate (PDR) BRT was used (10 Gy with dose rate of 0.4 Gy/h and 20 Gy with dose rate of 0.4 or 0.5 Gy/h); in the remaining cases high-dose rate (HDR) BRT was given with a mean dose of 16 Gy (range 8–25 Gy in 3–5 fractions) prescribed to 5 mm depth. In term of equivalent dose in 2 Gy fractions (EqD2), a dose between 18 and 20 Gy was administered in 7 cases, and a dose ranged between 9 and 14 Gy and between 23 and 31 Gy was administered in 4 cases and in 2 cases, respectively.
Median time gap between external RT and BRT was 21 days (range 12–53 days).
Treatment response and incidence of colostomy
At 6 months after the end of RT, 70 patients out of 84 patients (83%, 95% CI 74%–91%) had complete response (CR), 3 patients (4%) had partial response (PR), while 7 patients (8%) had progressive disease (3 in-field recurrence and 4 distant recurrence), 1 patient died from non-cancer-related causes, and 1 patient died for unknown reasons. For 2 patients, data were not available at 6 months analysis.
Of these, 74 patients remained for the analysis at 12 months follow-up: 60 (81%) (95% CI 70–89) patients had CR, no patients had PR, while 11 (15%) patients had progressive disease (6 in-field recurrence and 5 distant recurrence), 2 patients died from non-cancer-related causes, and 1 patient died for unknown cause. For 10 patients, data were not available at time of the analysis (6 patients have a follow-up shorter than 12 months; 4 patients were lost at follow-up).
Four patients had colostomy before the start of RT.
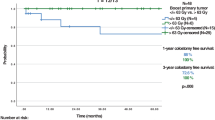
Six patients had colostomy in the first year after the start of RT (2 patients during RT and 4 patients after the end of RT), with an estimated cumulative incidence of colostomy at 6 and 12 months of 4% (95% CI 1–10) and 8% (95% CI 3–15), respectively (Fig. 1a); CFS was 96% (95% CI 89–99) at 6 months and 92% (95% CI 83–96) at 12 months (Fig. 1b). Two of these were disease-associated colostomies; the other 4 were treatment-associated.
Data on colostomy. a Cumulative incidence of colostomy during the first year of follow-up. b Colostomy-free survival during the first year of follow-up. * 80 patients entered the analysis. Four patients had colostomy before the start of RT. IC cumulative incidence of colostomy, CFS colostomy free survival, RT radiation therapy, m months, CI confidence interval
Compliance and interruptions
Table 2 shows compliance, reason of interruptions, and the incidence of acute and early-late toxicities.
Median RT duration time was 47.5 days (range 32–69 days).
A treatment interruption, due mainly for skin toxicity, occurred in 65 patients (77%); the median interruption within these patients was 7 days (range 1–21 days).
Analyzing interruption causes 41 patients suffered from skin toxicity (63%), 11 patients suffered from GI toxicity (17%), 3 patients experienced hematological toxicity (5%), and 1 patient (1%) suffered from GU toxicity.
The rest 14% of patients interrupted for the following reasons: 3 for local infections, 2 for patient choice, 2 for technical problems, 1 for allergic reaction to concomitant drug, and 1 for deterioration of Karnofsky Performance Status (KPS).
Toxicity
Acute events
Acute toxicity was recorded for the whole study group from the end of RT to 6 months.
Severe GI toxicity (≥ G3) was observed in 4 patients (5%). Severe skin toxicity occurred in 19 cases (23%).
Early-late events
Early-late toxicity was collected for 73 subjects (87%).
Severe GI toxicity (≥ G3), diarrhea, was observed in 2 patients (3%).
Severe vulvo-vaginal toxicity was observed in 2 (3%) patients.
No skin toxicity was reported.
No acute or early-late severe GU toxicity was reported at both time points.
As shown in Table 3, at univariate analysis, no statistically significant association was found between age, sex, HPV positivity, KPS, stage, and severe acute or early-late toxicity. A trend of correlation was reported between dose (< 56Gy/ = 56Gy/> 56Gy), severe acute toxicity (p value 0.058), and RT duration (< 47.5 days and ≥ 47.5 days) and severe early-late toxicity (p value 0.054).
In 70 patients treated with capecitabine plus cisplatin, G3–G4 hematologic toxicity was described in 9 cases (12.8%): neutropenia in 6 patients, anemia in 2 cases, and thrombocytopenia in one case.
Discussion
Innovative IMRT technique with daily IGRT enables precise conformation of the radiation dose to the target volume and sparing of normal tissues such as bowel, urinary bladder, femoral heads, external genitalia, and bones, thanks to the steep dose gradient resulting in dramatic dose falloffs [18]. Accordingly, it should be considered the standard of care in treating anal cancer patients [19].
In the present analysis, all patients were treated with IMRT; the acute and early-late non-hematologic toxicity profile of patients appeared to be excellent. In addition, the high rate of clinical tumor responses despite the inclusion of locally advanced stages of disease (stage ≥ III in 61% of cases) needs to be highlighted.
We have a high number of interruptions mainly due to skin toxicity. Literature has already demonstrated how IMRT can reduce acute toxicity without eliminating it [6,7,8,9,10,11,12,13,14].
The most common G3 or G4 toxicity in the two big trials which used 3-dimensional conformal radiotherapy (3D-CRT) was radiodermatitis. In RTOG 9811 [20] and ACT II trials, ≥ G3 skin toxicity was 48% in the standard mitomycin-based arm. In RTOG 0529, with IMRT, ≥ G3 skin toxicity was significantly reduced (23%) as well as the ≥ G3 GI events (21% vs. 36%; p = .008). The combined ≥ G2 acute adverse event rate was 77%, identical to the overall rate in RTOG 9811 (77%). Comparing our data to these, they look more alike the RTOG 9811, but if we consider skin acute toxicity at the end of RT treatment (22.6%) and the median overall treatment time (47.5 days), they are comparable to RTOG 0529, with a lower GU and GI toxicity. Several studies have showed that IMRT significantly lowers the probability of interruptions during the treatment compared to 3D-CRT technique. Even though the clinical benefit of interruption-free treatment is still under debate, the overall treatment time seems to impact on therapy response [21, 22]. In our series, skin toxicity played the major role in RT breaks (77%), but median overall treatment time (47.5 days) did not exceed the ideal duration of 53 days [23], and all the patients completed the planned treatments.
It is important to note that skin toxicity is mainly limited to the perineal area, and the perianal skin is often part of the CTV or falls into the PTV, making the dosimetric sparing challenging. However, perineal skin sparing should be done with caution and should not be given priority over CTV coverage [24].
No ≥ G3 GU toxicity was recorded in the first year of follow-up, and only 2 (3%) patient suffered from GI severe toxicity after 6 months of follow-up, underlining how the dosimetric organ sparing can actually be translated into clinical benefit; as concerns the increased local failure due to the risk of potential target missing, it found no confirmation in the dedicated literature [25, 26].
The incidence of the side effects found in the current study was comparable with that observed in other series. Call et al. reported an acute severe GI toxicity of 12% [27], which is higher than what we experienced, but a comparable acute severe skin toxicity of 20%. The same was found by Salama et al. who reported a severe skin toxicity of 37.7% [9]. In terms of colostomy, colostomy incidence was 4% at 6 months and 8% at 12 months; CFS at 6 months was 96.3% and at 12 months was 92.1% (Fig. 1a and b).
A comparison with literature data is difficult because in many papers, as well as the present one, CFS (both disease-associated and treatment-associated) is considered one parameter, and it does not differentiate between the two [28].
One reason may be because CFS has always been more linked to quality of patient’s life (that it is better without a colostomy regardless of its indication). Another explanation may be because the intervention can subjectively vary case by case (as, e.g., some patients refuse to have a stoma or demand one if late effects are severe), although it is intuitive that the clinical meaning of disease-associated versus treatment-associated colostomy differs greatly. This said, our data seem to compare favorably in the long term with recently published rates of 75.5% at 2 years [29, 30].
Currently, literature data are not conclusive about the modulation of dose in different disease stages. The randomized studies offered 45 Gy to the pelvis followed by a boost of 9–20 Gy to the GTV. Doses > 59 Gy in the combined ChT-RT treatment have not showed an additional benefit [31, 32].
In our series, the median total dose was 56 Gy. By grouping patients receiving less than, equal to, and more than 56 Gy, there was not any statistically significant difference in toxicity (p value 0.058 and 0.68 at the end of RT and at 12 months, respectively), but there was a trend indicating that a dose ≥ 56 Gy might be associated with higher severe acute toxicity.
In regard to the boost on macroscopic disease, at our institution, SIB strategy is mostly used when patients are treated with Tomotherapy®. With the introduction and the mandatory use of IMRT in the setting of SCAC, it became clear that the SIB strategy can reduce OTT. As a consequence, it has the theoretical advantage of improving local control and increasing patient’s compliance to treatment. Mild acute toxicity profile and limited long-term sequelae were found, confirming the feasibility and the effectiveness of SIB-IMRT, also when combined with concurrent ChT [33, 34].
However, both SIB and sequential boost approaches were used in this series, according to the data that both methods are effective treatment strategies in the combined modality therapy of anal cancer patients [35].
Thirteen patients underwent BRT as a boost, in the first years of IMRT implementation in our division of RT, following a clinical practice quite common at the time of 3D-CRT. BRT in anal cancers has been performed worldwide for nearly a century [36] and a number of reports compared outcome of external beam versus BRT boost [37, 38].
According to the data that the time gap between external RT and BRT boost are prognostic factors for the local control rate [39], only two patients received the BRT boost after a time gap > 1 month. One of them received the boost after 39 days because of skin and gastrointestinal toxicity; for another patient, a restaging MRI was needed to evaluate the response and the better boost modality to apply.
However, given the lack of prospective data and the improved toxicity profile of advanced external beam RT modalities, the use of BRT has been declining over time [40].
In our retrospective study we collected data of patients treated with different ChT schemes containing fluoropyrimidines (infusional 5-FU or oral capecitabine) plus mitomycin or mainly cisplatin. The role of cisplatin, well-known for its radiosensitizing properties, in patients with SCAC treated with curative intent is not yet entirely clarified, due to conflicting data about its direct comparison with mitomycin: different doses, different regimens, and different strategies were compared. But it seems more manageable due to lower hematologic toxicity [41, 42].
Limited data are also available regarding capecitabine combined with mitomycin or cisplatin in SCAC, even if this agent is worldwide recognized as a cost-effective alternative of infusional 5FU, also when concomitantly administered with radiotherapy. Literature data on prospective phase II or retrospective trials supported similar efficacy of oral fluoropyrimidine but better tolerability (less hematologic toxicity) and fewer radiotherapy interruptions [43,44,45,46,47].
As expected, G3–G4 hematologic toxicity in our series was limited (12.8% of patients treated with capecitabine plus cisplatin). It should be noted that bone marrow toxicity caused by combinations containing mitomycin is reported in literature in 30–60% of treated patients. Other detailed results regarding efficacy and toxicity of cisplatin-capecitabine regimen will be published soon [48].
Our series included also 4 patients with metastatic disease, who were locally treated with full-dose ChT-RT, because although prognosis is generally poor (10% of patients surviving ≥ 2 years), long-term survivors are described [49].
We decided to include these 4 patients in our analysis as the main goal was to evaluate local control and toxicity; in this sense, the presence of metastasis does not have an impact on our primary endpoints. Moreover, the improvement of OS and DFS in metastatic anal cancer patients who received radiation therapy is often reported in literature, suggesting a role for local treatment even in case of systemic disease [50].
In terms of outcome, local control was excellent with a CR rate of 83% and 81% at 6 and 12 months, respectively, becoming higher when considering partial and slow responders. Guidelines recommend a waiting time of 26 weeks to consider treatment failure, but 2 of the 3 patients with PR at 6 months achieved CR at 12 months, while the third one has been slowly responding as he gets close to 12-month follow-up.
SCAC may regress slowly after completion of radiotherapy treatment, so precise identification of tumor response is essential to optimize patient management.
Two patients with synchronous metastases had CR both locally and distally, after ChT-RT, highlighting the importance of obtaining complete local control even in the context of metastatic disease.
Although a longer follow-up is needed, in our study group, clinical outcome was excellent (PD rate of 8% and 15% at 6 and 12 months, respectively) and seemed superior to that reported in historical series, confirming the trend of anal cancer toward better prognosis over time [51].
Even though the variability in the ChT may have affected the toxicity, the response to treatment seemed to be equivalent, in terms of disease-free and/or CFS [52]. Our study has several limitations that should be noted. Firstly, its retrospective nature, secondly, a certain level of heterogeneity in the RT doses, treatment, and ChT regimens, which is the reason why we considered the median RT dose, and thirdly long-term chronic toxicity and treatment response were not analyzed and will be the object of a next study.
Our study showed an excellent clinical result and low acute toxicity rates, confirming the use of IMRT as standard of care for curative treatment of anal cancer patients.
References
Nigro ND, Vaitkevicius VK, Considine B (1974) Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 17:354–356
Cummings BJ, Keane TJ, O’Sullivan B et al (1991) Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys 21:1115–1125
Papillon J, Chassard JL (1992) Respective roles of radiotherapy and surgery in the management of epidermoid carcinoma of the anal margin. Series of 57 patients. Dis Colon Rectum 35:422–429
Uronis HE, Bendell JC (2007) Anal cancer: an overview. Oncologist 12:524–534
Pepek JM, Willett CG, Czito BG (2010) Radiation therapy advances for treatment of anal cancer. J Natl Compr Cancer Netw 8:123–129
Kachnic LA, Winter K, Myerson RJ et al (2013) RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 86(1):27–33
Milano MT, Jani AB, Farrey KJ et al (2005) Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 63:354–361
Mitchell MP, Abboud M, Eng C, Beddar AS, Krishnan S, Delclos ME, Crane CH, Das P (2014) Intensity-modulated radiation therapy with concurrent chemotherapy for anal cancer: outcomes and toxicity. Am J Clin Oncol 37:461–466
Salama JK, Mell LK, Schomas DA et al (2007) Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol 25:4581–4586
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version1.1). Eur J Cancer 45(2):228–247
Ng M, Leong T, Chander S, Chu J et al (2012) Australasian gastrointestinal trials group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys 83(5):1455–1462
ICRU Report 83. Prescribing, recording, and reporting intensity-modulated photon-beam therapy (IMRT) (ICRU Report 83). International Commission on Radiation Units and Measurements, Bethesda, MD (2010)
Menkarios C, Azria D, Laliberté B et al (2007) Optimal organ-sparing intensity-modulated radiation therapy (IMRT) regimen for the treatment of locally advanced anal canal carcinoma: a comparison of conventional and IMRT plans. Radiat Oncol 15(2):41
Viswanathan AN, Moughan J, Miller BE, Xiao Y, Jhingran A, Portelance L, Bosch WR, Matulonis UA, Horowitz NS, Mannel RS, Souhami L, Erickson BA, Winter KA, Small W Jr, Gaffney DK (2015) NRG oncology/ RTOG 0921: a phase 2 study of postoperative intensity-modulated radiotherapy with concurrent cisplatin and bevacizumab followed by carboplatin and paclitaxel for patients with endometrial cancer. Cancer. 121(13):2156–2163
Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI). Common Terminology Criteria for Adverse Events (CTCAE): version 4.0. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda: Maryland; 2009. v4.02: 2009
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Wiley & Sons Ltd; 1980
Dewas CV, Maingon P, Dalban C et al (2012) Does gap-free intensity modulated chemoradiation therapy provide a greater clinical benefit than 3D conformal chemoradiation in patients with anal cancer? Radiat Oncol 7:201
De Bari B, Lestrade L, Franzetti-Pellanda A et al (2018) Modern intensity-modulated radiotherapy with image guidance allows low toxicity rates and good local control in chemoradiotherapy for anal cancer patients. J Cancer Res Clin Oncol 144(4):781–789
Ajani JA, Winter KA, Gunderson LL et al (2006) Intergroup RTOG 98–11: a phase III randomized study of 5-fluorouracil (5-FU), mitomycin, and radiotherapy versus 5-fluorouracil, cisplatin and radiotherapy in carcinoma of the anal canal. J Clin Oncol 24(18_suppl):4009–4009
Chuong M, Freilich J, Hoffe S, Fulp W, Weber JM, Almhanna K, Dinwoodie W, Rao N, Meredith KL, Shridhar R (2013) Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Gastrointest Cancer Res 6:39–45
Glynne-Jones R, Sebag-Montefiore D, Adams R et al (2011) “Mind the gap”—the impact of variations in the duration of the treatment gap and overall treatment time in the first UK anal cancer trial (act I). Int J Radiation Oncology Biol Phys 81(5):1488–1494
Ben-Josef E, Moughan J, Ajani JA, Flam M, Gunderson L, Pollock J, Myerson R, Anne R, Rosenthal SA, Willett C (2010) Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of radiation therapy oncology group trials 87-04 and 98-11. J Clin Oncol 28(34):5061–5066
Dell’Acqua V, Kobiela J, Kraja F et al (2018) Genital marginal failures after intensity-modulated radiation therapy (IMRT) in squamous cell anal cancer: no higher risk with IMRT when compared to 3DCRT. Med Oncol 35:59
Chen AM, Farwell DG, Luu Q et al (2011) Marginal misses after post- operative intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 80(5):1423–1429
Koeck J, Lohr F, Buergy D et al (2016) Genital invasion or perigenital spread may pose a risk of marginal misses for intensity modulated radiotherapy (IMRT) in anal cancer. Radiat Oncol 4(11):53
Call JA, Prendergast BM, Jensen LG et al (2016) Intensity-modulated radiation therapy for anal cancer: results from a multi-institutional retrospective cohort study. Am J Clin Oncol 39(1):8–12
Bazan JG, Hara W, Hsu A, Kunz PA, Ford J, Fisher GA, Welton ML, Shelton A, Kapp DS, Koong AC, Goodman KA, Chang DT (2011) Intensity modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer. 117:3342–3351
Glynne-Jones R, Kadalayil L, Meadows HM, Cunningham D, Samuel L, Geh JI, Lowdell C, James R, Beare S, Begum R, Ledermann JA, Sebag-Montefiore D, ACT II Study Group (2014) Tumour- and treatment-related colostomy rates following mitomycin C or cisplatin chemoradiation with or without maintenance chemotherapy in squamous cell carcinoma of the anus in the ACT II trial. Ann Oncol 25(8):1616–1622
Faivre JC, Peiffert D, Vendrely V, Lemanski C, Hannoun-Levi JM, Mirabel X, Stanbury T, Salleron J, Guillemin F (2018) Prognostic factors of colostomy free survival in patients presenting with locally advanced anal canal carcinoma: a pooled analysis of two prospective trials (KANAL 2 and ACCORD 03). Radiother Oncol 129(3):463–470
Hosni A, Han K, Le Lisa W et al (2018) The ongoing challenge of large anal cancers: prospective long term outcomes of intensity-modulated radiation therapy with concurrent chemotherapy. Oncotarget. 9(29):20439–20450
John M, Pajak T, Flam M et al (1996) Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am 2(4):205–211
Tomasoa NB, Meulendijks D, Nijkamp J, Cats A, Dewit L (2016) Clinical outcome in patients treated with simultaneous integrated boost - intensity modulated radiation therapy (SIB-IMRT) with and without concurrent chemotherapy for squamous cell carcinoma of the anal canal. Acta Oncol 55(6):760–766
Arcadipane F, Franco P, Ceccarelli M et al (2018) Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia Pac J Clin Oncol 14(3):217–223
Franco P, De Bari B, Arcadipane F et al (2018) Comparing simultaneous integrated boost vs sequential boost in anal cancer patients: results of a retrospective observational study. Radiat Oncol 13(1):172
Dalby JE, Pointon RS (1961) The treatment of anal carcinoma by interstitial irradiation. Am J Roentgenol Radium Therapy, Nucl Med 85:515–520
Oehler-Jänne C, Seifert B, Lütolf UM et al (2007) Clinical outcome after treatment with brachytherapy boost versus external beam boost for anal carcinoma. Brachytherapy. 6(3):218–226
Moureau-Zabotto L, Ortholan C, Hannoun-Levi JM et al (2013) Role of brachytherapy in the boost management of anal carcinoma with node involvement (CORS-03 study). Int J Radiat Oncol Biol Phys 85(3):e135–e142
Niehoff P, Kovács G (2014) HDR brachytherapy for anal cancer. J Gastrointest Oncol 5(3):218–222
Shridhar R, Shibata D, Chan E et al (2015) Anal cancer: current standards in care and recent changes in practice. CA Cancer J Clin 65(2):139–162
Gunderson LL, Winter KA, Ajani JA et al (2012) Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 30:4344–4351
James RD, Glynne-Jones R, Meadows HM et al (2013) Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol 14:516–524
Goodman KA, Julie D, Cercek A et al (2017) Capecitabine with mitomycin reduces acute hematologic toxicity and treatment delays in patients undergoing definitive Chemoradiation using intensity modulated radiation therapy for anal Cancer. Int J Radiat Oncol Biol Phys 98:1087–1095
Glynne-Jones R, Meadows H, Wan S et al (2008) EXTRA-A multicenter phase II study of Chemoradiation using a 5 day per week Oral regimen of capecitabine and intravenous mitomycin C in anal Cancer. Int J Radiat Oncol Biol Phys 72:119–126
Thind G, Johal B, Follwell M et al (2014) Chemoradiation with capecitabine and mitomycin-C for stage I-III anal squamous cell carcinoma. Radiat Oncol 9:124
Jones CM, Adams R, Downing A et al (2018) Toxicity, tolerability, and compliance of concurrent capecitabine or 5-fluorouracil in radical management of anal cancer with single-dose mitomycin-C and intensity modulated radiation therapy: evaluation of a national cohort. Int J Radiat Oncol Biol Phys 101:1202–1211
Eng C, Chang GJ, You YN et al (2013) Long-term results of weekly/daily cisplatin-based chemoradiation for locally advanced squamous cell carcinoma of the anal canal. Cancer 119:3769
Souza K, Pereira A, Araujo R et al (2016) Replacing 5-fluorouracil by capecitabine in localised squamous cell carcinoma of the anal canal: systematic review and meta-analysis. Ecancermedicalscience. 10:699
Glynne-Jones R, Nilsson PJ, Aschele C et al (2014) Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 40(10):1165–1176
Repka M, Aghdam N, Karlin A et al (2017) Social determinants of stage IV anal cancer and the impact of pelvic radiotherapy in the metastatic setting. Cancer Med 6(11):2497–2506
Elson JK, Kachnic LA, Kharofa JR (2018) Intensity-modulated radiotherapy improves survival and reduces treatment time in squamous cell carcinoma of the anus: a National Cancer Data Base Study. Cancer. 124(22):4383–4392
Flam M, John M, Pajak TF et al (1996) Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase II randomized intergroup study. J Clin Oncol 14:2526–2539
Funding
This study was partially supported by Fondazione IEO-CCM, project title: “AXILL-ART: Biology-based radiotherapy volume definition for 1-2 macrometastatic sentinel lymph nodes without further dissection in breast cancer conservative surgery” and by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design in terms of material preparation, data collection, and analysis. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (research project entitled “Image guided radiotherapy in gastrointestinal malignancies” notified to the Ethical Committee of the IEO, Milan, Italy No. IEO N87/11) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Veronica Dell’Acqua and Alessia Surgo are the Co-first author.
Maria Cristina Leonardi and Barbara Alicja Jereczek-Fossa are the Co-last author.
Rights and permissions
About this article
Cite this article
Dell’Acqua, V., Surgo, A., Arculeo, S. et al. Intensity-modulated radiotherapy (IMRT) in the treatment of squamous cell anal canal cancer: acute and early-late toxicity, outcome, and efficacy. Int J Colorectal Dis 35, 685–694 (2020). https://doi.org/10.1007/s00384-020-03517-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03517-x