Abstract
Purpose
Primary local excision (PLE) for early rectal cancers is associated with decreased surgical morbidity and mortality compared with major resection (MR). However, it is thought to be associated with poorer oncological outcomes. There is a paucity of data regarding PLE within the Australasian population. We present comparative post-operative and survival outcomes for stage 1 rectal cancers treated with PLE or MR from three Western Australian hospitals.
Methods
A retrospective analysis was performed on a prospectively maintained database of patients undergoing PLE or MR for stage 1 rectal cancers between February 1996 and May 2019.
Results
Of the 533 patients, 81 underwent PLE. Median post-operative admission was shorter for those undergoing PLE, with no significant difference in post-operative complication rate. Five-year overall survival was greater following MR (89.6% CI 86.1–92.3) compared with PLE (84.6% CI 73.8–91.2; p = 0.0003). There was no significant difference in 5-year cancer-specific survival (MR, 94.4% CI 91.5–96.3; PLE, 95.3% CI 86.0–98.5; p = 0.98) or 5-year disease-free survival (MR, 92.3% CI 89.1–94.7; PLE, 89.1% CI 78.5–94.7; p = 0.36). Local excision provided poorer local tumour control with an inferior 5-year local recurrence rate (MR, 2.16% CI 1.08–4.28; PLE, 10.9% CI 5.30–21.6; p = 0.0002). After controlling for confounders, PLE was significantly associated with worse local recurrence but did not significantly impact overall survival, cancer-specific survival, overall recurrence, or metastatic recurrence.
Conclusion
Local excision of early rectal cancer remains a viable alternative, in those unwilling or unable to undergo MR. Patients should be informed that while PLE is associated with poorer local pelvic control, this does not translate to worse survival.
Trial Registration
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer, one of the leading causes of cancer-related mortality, has historically been treated with major surgical resection. There is, however, a growing trend to utilise local excision techniques. While surgical technique and peri-operative management of patients have vastly improved over time, many patients do not have the physiological reserve to undergo major resection (MR). Furthermore, many patients are reluctant to undergo these procedures due to the risk of long-term sexual, urinary and bowel functional impairment as well as the risk of temporary or permanent stoma [1]. In these instances, local excision may facilitate resection. This surgical approach does not allow for adequate resection of the mesorectal tissue and as such is oncologically unsuitable for advanced disease and risks failing to identify occult lymph node involvement in early disease [2, 3]. Given likelihood of nodal involvement is determined by depth of invasion, local excision of rectal cancers is supported for tumours invading the submucosa only [4,5,6]. The peri-operative benefits of local excision techniques compared with MR for rectal cancer are well reported in the literature [7]. Recent large-scale studies have demonstrated local excision to provide comparable survival to MR but poorer local tumour control [8]. However, to date, these results have not been replicated within an Australasian population.
The aim of this study is to compare peri-operative and oncological outcomes of stage 1 rectal cancer treated with primary local excision (PLE) and MR.
Methods
A retrospective analysis was performed on a prospectively maintained database of all patients with colorectal cancer attending three hospitals in Perth, Western Australia. The database contains demographic, histopathological, peri-operative and long-term survival data from colorectal cancer patients diagnosed between February 1996 and May 2019. Patient contribution to the database is voluntary, and their consent to participate was obtained in the pre-operative setting.
Patients were considered for PLE if they had stage 1 rectal cancer or advanced rectal cancer in a patient unsuitable or unwilling to undergo MR. The decision to proceed to MR versus PLE was collaboratively reached by patient and clinician, following discussion at multidisciplinary clinicopathology meeting. Patients were reviewed post-operatively at 2 weeks and subsequently underwent endoscopic and/or multiplanar imaging at six monthly intervals post-procedure. Patients undergoing PLE underwent flexible sigmoidoscopy more frequently than the MR cohort, at 6 months, 1 year and 2 years post-procedure.
Definitions
Date of diagnosis was defined as the date of histopathological diagnosis of rectal adenocarcinoma. The American Society of Anesthesia (ASA) score was used as a marker of overall health at surgery. Neoplasia was staged and graded according to current American Joint Committee on Cancer (AJCC) staging guidelines [9]. Major resections included abdominoperineal resection, anterior resection, Hartmann’s operation or other procedures (total or subtotal colectomy and proctectomy). Local excision procedures included transanal endoscopic microsurgery (TEM) or transanal excision (TAE). Patients were considered part of the PLE cohort if they underwent a local excision procedure without progression to a MR. Resection without macroscopically detectable disease was considered curative (R0 and R1). Tumour height was measured in centimetres from the anal verge.
Peri-operative outcomes included (1) post-operative length of admission in days, (2) unplanned return to theatre (reoperation within 30 days of the initial procedure due to surgical complications), (3) intra-abdominal infection (demonstrated on imaging, visualised at re-operation, or on aspiration of purulent or microbiologically positive material) and (4) incidence of post-surgical procedural interventions (unplanned intensive care unit admission, blood product transfusion, radiological drainage of a collection).
Overall recurrence was defined by the presence of either (1) local pelvic recurrence or (2) distant recurrence of metastasis not present at the time of initial diagnosis.
Inclusion and exclusion criteria
Patients were included in the study if they met the following inclusion criteria: (1) tumour located in the rectum, (2) histologically confirmed adenocarcinoma, (3) stage 1 disease and (4) underwent either MR or PLE. Patients were excluded from the study if they did not undergo resection with curative intent.
The database contains very few patients with stage 2 rectal cancer that underwent PLE (n = 6). Within this cohort, there was only one incident of recurrence (distant metastases) with subsequent cancer-related mortality (at 2.4 years following diagnosis). This cohort size does not provide sufficient power to detect statistically significant differences between MRE and PLE for stage 2 disease. Accordingly, this study was restricted to patients with stage 1 rectal cancer.
Statistics
Data was prospectively entered into a database (Filemaker Pro 15; Filemaker, Santa Clara, CA, USA) and then exported to a statistical package for analysis (Stata 15; Statacorp, College Station, TX, USA). Median and interquartile range (IQR) were used for descriptive statistics. Nominal data were compared using Pearson’s chi-square test. Ordinal data and continuous non-parametric data were compared using Mann-Whitney U test, while continuous parametric data were compared using Student’s t test. Normal distribution of data was assessed using the Shapiro-Wilk test. Survival data was analysed using the Kaplan-Meier estimator with subsequent log-rank test for equality of survival functions and the Cox proportional hazard regression analysis. Recurrence rates were analysed using cumulative incidence probability. Survival analysis was truncated to the point when the number at risk was one-third of the starting figure. Survival and recurrence data were obtained through clinician review and annual review of the Western Australia Cancer Registry, which reports survivorship, cause of death and recurrence. All survival and recurrence times were calculated from the date of histological diagnosis until either an event occurred or they remained alive at 09 October 2018. Statistical significance was defined p < 0.05. Confidence intervals (CI) were set at the 95% level.
Results
There were 533 patients that fit the eligibility criteria for this study. The median age was 65 years (IQR 57–75 years), and the female to male ratio was 1:1.65. The MR cohort includes 43 patients that required salvage surgery following PLE (TEM, n = 32; TAE, n = 11). The patients in this group that underwent TEM did not have tissue-confirmed malignancy at the time of initial procedure, and thus progressed to MR upon histological recognition of malignancy. Twenty-four of these patients underwent anterior resection (ultra-low anterior resection, n = 22; high anterior resection, n = 2), eight underwent abdominoperineal resection, one underwent a Hartmann’s procedure and ten patients had other major surgical resections. Of these, four patients developed recurrence, only one of which died due to cancer at 18.2 months following initial surgery. The median time to recurrence in those requiring salvage surgery was 17.6 months (IQR 8.04–34.1 months).
Clinicopathological characteristics
Characteristics of patient, tumour and treatments are described in Table 1 and Fig. 1.
Of the patients undergoing PLE, 66 patients underwent TEM, 13 patients underwent TAE and two underwent colonoscopic polypectomy.
There were 199 patients that were downstage to stage 1 following neo-adjuvant radiotherapy (stage 2, n = 62; stage 3, n = 137). Only one of these patients, who was originally stage 3, underwent PLE.
Peri-operative outcomes
The median length of admission for patients undergoing MR was 10 days (IQR 8–13 days). This was significantly longer than those undergoing PLE (2 days, IQR 1–3 days; p < 0.0001). There was a non-significant difference in the rates of post-procedural intra-abdominal abscess (MR, 1.33% n = 6; PLE, 0.00% n = 0; p = 0.3). All incidences of unplanned return to theatre and post-surgical interventional procedures occurred in the MR group. There were 19 (4.40%) unplanned returns to theatre and 20 (4.42%) interventional procedures post-surgery. These differences were not statistically significant between the cohorts (unplanned return to theatre, p = 0.06; post-procedural intervention, p = 0.054). Half of the patients returning to theatre (n = 10) had interventional procedures following surgery.
Oncological outcomes
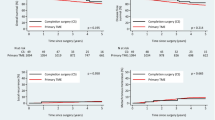
The median follow-up time after MR and PLE was 6.9 years (IQR 3.0–10.7 years) and 5.7 years (IQR 4.0–10.6 years), respectively. Follow-up was truncated at 10 years when the number at risk was one-third of the starting cohort. Table 2 contains the cohort-specific 5-year survival and recurrence rates. Figure 2 displays the overall survival and cancer-specific survival curves. The cumulative incidence of local recurrence and new metastases, as well as disease-free survival, are shown in Fig. 3. The multivariate regression models of overall survival, cancer-specific survival, disease-free survival and local and metastatic recurrence are displayed in Tables 3 and 4. There were no significant interactions between variables for any regression endpoints. After adjusting for confounding factors, PLE was not associated with a significant difference in overall survival, cancer-specific survival, disease-free survival or development of metastatic recurrence. Once confounding factors were controlled for, PLE was significantly associated with only increased rates of local recurrence. These confounding factors, displayed in Tables 3 and 4, included age at diagnosis, ASA score, lymphovascular invasion and the use of adjunct therapies.
Discussion
This study reviewed oncological and peri-operative outcomes following treatment of stage 1 rectal cancer with either PLE or MR within an Australasian population. Primary local excision facilitated resection in an older and more comorbid population, with tumours located lower in the rectum. Primary local excision was associated with a significantly shorter post-operative hospital admission, with a non-significant difference in rates of peri-operative complications. The PLE cohort had comparable cancer-specific and disease-free survival, but inferior overall survival, compared with the MR cohort. When confounding factors were controlled for, operative management with either PLE or MR did not have a significant effect on most long-term oncological outcomes but was associated with higher rates of local recurrence.
Our findings regarding peri-operative outcomes conflict with large-scale reviews demonstrating more frequent complications following MR [7, 10,11,12]. This is likely due to their more inclusive definition of significant peri-operative complication [10,11,12]. The publications that found comparable rates of peri-operative complications, including our own, have relatively low incidence of complications and small sample sizes, which are underpowered to demonstrate significant differences [13]. The prolonged length of post-operative admission following MR compared with PLE is in keeping with the literature [10,11,12, 14].
Recent analysis of the National Cancer Database (NCDB) shows a contemporary increase in utilisation of local excision techniques for stage 1 rectal cancer [15]. Comparatively, our rate of T1 tumours treated with PLE is only two-thirds than that of the NCDB and less than half their reported rate of T2 tumours treated with PLE. In keeping with our findings, this large-scale review reported a higher rate of positive resection margins following PLE compared with MR (24% vs 5%). Several smaller scale studies have confirmed that PLE is associated with higher rates of positive resection margins [14, 16, 17]. The NCDB study, while not differentiating between macroscopic or microscopic margins, found that a positive resection margin was independently associated with worse overall survival. In our study, R1 resection margin was not associated with worse oncological outcomes. Despite this, the authors of this paper agree that patients with positive resection margins, following local excision of early rectal cancer, should undergo transabdominal resection or adjuvant therapy if unsuitable for surgery [18, 19].
It is widely accepted that patients undergoing PLE have poorer overall survival compared with those that undergo MR [7, 8]. This likely reflects disparities in age, baseline comorbid disease and tumour invasion status as far greater predictors of overall survival than treatment modality [10, 15, 20]. Similarly, the difference in overall survival that we have reported is best described by differences in patient frailty, represented by patient age and ASA. This reflects surgeon bias toward PLE in older and more comorbid patients.
The increased uptake in local excision techniques has occurred despite poorer local tumour control. The Swedish Rectal Cancer Registry 5-year local recurrence rate for stage 1 rectal cancer treated with PLE was 11.2% and 2.2% following MR [21]. This compares with NCDB figures of 12.5% for T1 tumours and 22.1% for T2 tumours treated with PLE [10]. This large-scale publication reports a 5-year local recurrence rate following MR of 2.2% for T1 tumours and 15.1% for T2 tumours. Our 5-year local recurrence rates of 10.9% (CI 5.30–21.6%) for PLE and 2.16% (CI 1.08–4.28%) for MR are not dissimilar to these reported figures. Despite poorer local tumour control, paradoxically, we report a similar 5-year cancer-specific survival following PLE (95.3% CI 86.0–98.5) and MR (94.4% CI 91.5–96.3), mirroring findings of recent publications [7, 10, 12, 22, 23]. In those suitable, salvage surgery remains an oncologically viable option for patients with local recurrence following PLE [18].
The presence of lymphovascular invasion significantly influenced all survival endpoints. Lymphovascular invasion is a well-reported marker of adverse prognosis for rectal cancer. Furthermore, several large-scale publications have found lymphovascular invasion, along with perineural invasion, and advanced tumour grade and invasion status to be associated with higher rates of disease recurrence following local excision [10, 14, 24, 25]. Due to low incidence of perineural invasion and lack of heterogeneity of tumour grade and invasion status, the current dataset is underpowered to adequately detect the influence of these factors within the PLE cohort.
While not reflected by our study’s findings, it is thought that PLE alone provides inferior disease-free survival compared with PLE combined with adjunct chemoradiation therapy for T1 tumours with adverse prognostic features and T2 tumours [26,27,28]. In these cohorts, suitable patients should be considered for primary MR or salvage surgery. Alternatively, if they are unwilling or unable to undergo MR, oncological outcomes are improved through the addition of adjunct chemoradiation therapy [26,27,28]. These patients need to be counselled regarding the risks of chemoradiotherapy, as well as the challenging nature of surgery following pelvic irradiation.
Our database is currently too small to perform meaningful subset analysis in regard to the combined effect of tumour invasion status, lymphovascular invasion and adjunct therapy on survival in the PLE cohort. As such, the benefit of combined PLE and chemoradiation therapy compared with PLE alone was not detected in this study. As our database continues to grow and local excision techniques continue to gain popularity, it is likely that we will be able to report on these variables.
The strengths of this retrospective review lie in the size of the patient population and their prolonged follow-up period. A strong partnership with the Western Australia Cancer Registry and relatively minor migration patterns have allowed for 100% patient follow-up and no patient dropout. Additionally, this database contains patients from multiple institutions and multiple surgeons, limiting the homogeneity of the group.
Our study was limited by its retrospective nature and the selection bias toward local excision for older and more comorbid patients. We utilised multivariate regression analysis to mitigate this effect, as the current dataset is underpowered to perform statistical matching techniques. This selection bias reflects evidence-based practise and is in keeping with global practise patterns of PLE for more frail and comorbid patients. The cohort contains a relatively small population of T2 tumours and T1 tumours with adverse prognostic features. As such, it is underpowered to provide subgroup analysis. The current database does not record specific factors influencing decisions regarding adjunct therapies. These can only be inferred, on a case-by-case basis, from recorded negative prognostic features.
Conclusion
Primary local excision remains a viable oncological alternative in those with early stage rectal cancer that are unsuitable or unwilling to undergo MR. While PLE provides poorer local tumour control, this does not translate to worse overall survival, cancer-specific survival or disease-free survival, when confounding factors are accounted for.
References
Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, Norton C, Hughes R, Laurberg S, Moran BJ (2016) Predicting the risk of bowel-related quality-of-life impairment after restorative resection for rectal cancer: a multicenter cross-sectional study. Dis Colon Rectum 59(4):270–280
Garcia-Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA (2000) Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg 231(3):345–351
Winde G, Nottberg H, Keller R, Schmid KW, Bünte H (1996) Surgical cure for early rectal carcinomas (T1). Dis Colon rectum 39(9):969–976
Sengupta S, Tjandra JJ (2001) Local excision of rectal cancer: what is the evidence? Dis Colon rectum 44(9):1345–1361
Brodsky JT, Richard GK, Cohen AM, Minsky BD (1992) Variables correlated with the risk of lymph node metastasis in early rectal cancer. Cancer 69(2):322–326
Morson BC (1966) Factors influencing the prognosis of early cancer of the rectum. Proc R Soc Med 59(7):607–608
Kidane B, Chadi SA, Kanters S, Colquhoun PH, Ott MC (2015) Local resection compared with radical resection in the treatment of T1N0M0 rectal adenocarcinoma: a systematic review and meta-analysis. Dis Colon rectum 58(1):122–140
Halverson AL, Morris AM, Cleary RK, Chang GJ (2019) For patients with early rectal cancer, does local excision have an impact on recurrence, survival, and quality of life relative to radical resection? Ann Surg Oncol 26:2497–2506
AJCC Cancer staging manual (2017). Cancer staging manual., 8th edn. Springer International Publishing,
You NY, Baxter NN, Stewart NA, Nelson NH (2007) Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg 245(5):726–733
Ptok H, Marusch F, Meyer F, Schubert D, Koeckerling F, Gastinger I, Lippert H (2007) Oncological outcome of local vs radical resection of low-risk pT1 rectal cancer. Arch Surg 142(7):649–654
De Graaf EJR, Doornebosch PG, Tollenaar RAEM, Meershoek-Klein Kranenbarg E, de Boer AC, Bekkering FC, van de Velde CJH (2009) Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 35(12):1280–1285
Lezoche E, Baldarelli M, Lezoche G, Paganini A, Gesuita R, Guerrieri M (2012) Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 99(9):1211–1218
Elmessiry MM, Van Koughnett JAM, Maya A, Dasilva G, Wexner SD, Bejarano P, Berho M (2014) Local excision of T1 and T2 rectal cancer: proceed with caution. Color Dis 16(9):703–709
Stitzenberg KB, Sanoff HK, Penn DC, Meyers MO, Tepper JE (2013) Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol 31(34):4276–4282
Allaix ME, Arezzo A, Giraudo G, Morino M (2012) Transanal endoscopic microsurgery vs. laparoscopic total mesorectal excision for T2N0 rectal cancer. J Gastrointest Surg 16(12):2280–2287
Endreseth BH, Myrvold HE, Romundstad P, Hestvik UE, Bjerkeset T, Wibe A, Group oboTNRC (2005) Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon rectum 48 (7):1380–1388
Morino M, Risio M, Bach S, Beets-Tan R, Bujko K, Panis Y, Quirke P, Rembacken B, Rullier E, Saito Y, Young-Fadok T, Allaix ME (2015) Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 29(4):755–773
Hahnloser D, Wolff BG, Larson DW, Ping J, Nivatvongs S (2005) Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon rectum 48(3):429–437
Bhangu A, Brown G, Nicholls R, Wong J, Darzi A, Tekkis P (2013) Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a surveillance, epidemiology, and end results (SEER) population-based study. Ann Surg 258(4):563–571
Saraste D, Gunnarsson U, Janson M (2013) Local excision in early rectal cancer-outcome worse than expected: a population based study. Eur J Surg Oncol 39(6):634–639
Hazard LJ, Shrieve DC, Sklow B, Pappas L, Boucher KM (2009) Local excision vs. radical resection in T1-2 rectal carcinoma: results of a study from the surveillance, epidemiology, and end results (SEER) registry data. Gastrointest Cancer Res 3(3):105–114
Folkesson J, Johansson R, Påhlman L, Gunnarsson U (2007) Population-based study of local surgery for rectal cancer. Br J Surg 94(11):1421–1426
Peng J, Chen W, Sheng W, Xu Y, Cai G, Huang D, Cai S (2011) Oncological outcome of T1 rectal cancer undergoing standard resection and local excision. Colorect Dis 13(2):14–19
Bach S, Hill J, Monson J, Simson J, Lane L, Merrie A, Warren B, Mortensen NM (2009) A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg 96(3):280–290
Borstlap WAA, Coeymans TJ, Tanis PJ, Marijnen CAM, Cunningham C, Bemelman WA, Tuynman JB (2016) Meta-analysis of oncological outcomes after local excision of pT1–2 rectal cancer requiring adjuvant (chemo) radiotherapy or completion surgery. Br J Surg 103(9):1105–1116
Chakravarti A, Compton CC, Shellito PC, Wood WC, Landry J, Machuta SR, Kaufman D, Ancukiewicz M, Willett CG (1999) Long-term follow-up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg 230(1):49–54
Lee L, Kelly J, Nassif GJ, Atallah SB, Albert MR, Shridhar R, Monson JR (2017) Chemoradiation and local excision for T2N0 rectal cancer offers equivalent overall survival compared to standard resection: a National Cancer Database analysis. J Gastrointest Surg 21(10):1666–1674
Acknowledgements
The authors would like to thank Richard Trevithick of the Western Australian Cancer Registry for his assistance in providing mortality data and death coding for the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen, R., Platell, C. Primary local excision of stage 1 rectal cancer is not associated with worse oncological outcomes when compared with major resection. Int J Colorectal Dis 35, 607–614 (2020). https://doi.org/10.1007/s00384-020-03512-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03512-2