Abstract
Purpose
The introduction of transanal endoscopic or minimally invasive surgery has allowed organ preservation for rectal tumors with good oncological results. Data on functional and quality-of-life (QoL) outcomes are scarce and controversial. This systematic review sought to synthesize fecal continence, QoL, and manometric outcomes after transanal endoscopic microsurgery (TEM) or transanal minimally invasive surgery (TAMIS).
Methods
A systematic review of the literature including Medline, Embase, and the Cochrane Library databases was conducted searching for articles reporting on functional outcomes after TEM or TAMIS between January 1995 and June 2018. The evaluated outcome parameters were pre- and postoperative fecal continence (primary endpoint), QoL, and manometric results. Data were extracted using the same scales and measurement units as from the original study.
Results
A total of 29 studies comprising 1297 patients were included. Fecal continence outcomes were evaluated in 23 (79%) studies with a wide variety of assessment tools and divergent results. Ten studies (34%) analyzed QoL changes, and manometric variables were assessed in 15 studies (51%). Most studies reported some deterioration in manometric scores without major QoL impairment. Due to the heterogeneity of the data, it was not possible to perform any pooled analysis or meta-analysis.
Conclusions
These techniques do not seem to affect continence by themselves except in minor cases. The possibility of worsened function after TEM and TAMIS should not be underestimated. There is a need to homogenize or standardize functional and manometric outcomes assessment after TEM or TAMIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Management of rectal lesions has radically evolved over the last decades. For locally advanced rectal cancer, the implementation of neoadjuvant chemo- and radiotherapy combined with total mesorectal excision raised the number of sphincter-saving procedures, but concern exists about the subsequent functional results [1,2,3,4,5]. On the other hand, full- or partial-thickness excision of the rectal wall with organ preservation can be sufficient to achieve oncologic clearance. This avoids the potential morbidity of pelvic surgery which involves anterior rectal resection, thus obtaining presumably better functional results.
In order to provide better access to the difficult rectal anatomy, transanal endoscopic microsurgery (TEM) was first introduced back in 1984 [6]. Later, the transanal endoscopic operation (TEO) was developed as a modification of TEM to treat rectal tumors. These two techniques are based on the use of a beveled rigid proctoscope attached to specialized pieces for insufflation in order to access rectal lesions with optimal visibility. More recently, transanal minimally invasive surgery (TAMIS) was introduced as an alternative to TEM and TEO, using a flexible and disposable single-port laparoscopic entry platform. TAMIS offers good tumor visualization with better versatility than the other transanal techniques since patient position is not influenced by tumor localization [7]. Compared with standard transanal excision, TEM has been associated with more intact, nonfragmented specimens (63% vs 100%), resulting in higher rates of negative resection margins (78% vs 98%) and lower rates of local recurrences (24% vs 8%) [8]. Similar findings have been reported for TAMIS, with R+ resection, fragmentation, and recurrence rates not exceeding 6%, 4%, and 2%, respectively [9]. Therefore, even if indications to TEM or TAMIS are similar to those of local excision, the former techniques are preferable in terms of visualization, exposure, and oncologic outcomes. Due to the evolution of minimally invasive instrumentation and advances in technologies, indications to transanal minimally invasive surgery have been expanded to different conditions, e.g., patients with advanced rectal lesions (T3) not fit to undergo major surgery, after chemoradiation therapy, or cT0 lesions after neoadjuvant chemotherapy (to confirm ypT0) [10, 11]. Indeed, the adoption of minimally invasive approach has rapidly grown worldwide during the recent years [10, 12].
Even though there is preservation of the rectum when these techniques are recommended, rectal and anal stretching produced by the introduction of a wide proctoscope or platform during the surgery, as well as partial organ resection, might provoke potential postoperative functional disorders such as fecal incontinence with consequent impairment of quality of life (QoL) [13, 14].
The aim of this systematic review is to synthesize the current evidence regarding continence, manometric, and QoL outcomes after TEM or TAMIS approaches for rectal lesions.
Methods
This systematic review of the literature was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15]. Aim of the review was to identify the functional outcome and quality of life after minimally invasive transanal surgery with organ preservation.
Search strategy
Medline, Embase, and the Cochrane Library databases were reviewed for articles published between January 1995 and June 2018. Search terms included “Transanal Endoscopic Surgery,” “Transanal Endoscopic Microsurgery,” “transanal minimally invasive surgery,” “transanal endoscopic operation,” “Rectal Neoplasms,” “rectal tumor*,” “rectal tumor*,” “rectal cancer*,” “rectal polyp*,” and “rectal lesion*.” The items were used in all possible combinations with the Boolean AND/OR to retrieve maximal number of articles. Exploded MeSH terms were included in the search strategy. English language restriction was imposed. Search strings for each database are attached as supplementary material. Additional articles by manually searching the reference lists from recent reviews and the extracted papers were also included. Detailed search strategy is reported in Appendix.
Study selection and quality assessment
Only studies reporting on pre- and postoperative functional outcomes of TEM or TAMIS surgery for rectal polyps or early rectal cancer were considered for inclusion. Outcomes of interest included the following: continence, quality of life, and manometric results. Studies reporting on low anterior resection of the rectum, local excision without endoscopic or minimally invasive techniques, or those only reporting on oncological outcomes, were excluded. Letters, guidelines, and systematic reviews were examined for cross-referencing and descriptive reasons, but not for data extraction. Studies were restricted to have a cohort of at least five patients. When the same cohort of patients was reported in different articles, only the article with the largest data and longest follow-up was selected. Inclusion and exclusion criteria are summarized in Table 1.
The initial assessment by title and abstract highlighted studies of potential interest for posterior full-text analysis. A full-text copy of each paper was reviewed by two authors independently (AC and FM), and data were retrieved according to these criteria. A discussion among all authors was established in case of any discrepancies. In case data were missing on included studies, authors were contacted in order to retrieve the respective information. In case there was no answer, those papers were excluded. Also, studies were discarded if TEM or TAMIS was associated to any kind of colectomies during the same operating act.
In order to assess methodological quality, both reviewers evaluated each article using the Newcastle-Ottawa Scale [16]. This scale is a validated and reliable tool for quality assessment of observational studies in systematic reviews. It comprises the evaluation of patient selection, comparability of cohorts, and outcomes. A score higher than five points was required for study inclusion.
Data extraction and analysis
Data of selected papers were extracted using a structured form which included year of publication, authors, number of patients, baseline demographics, technique, and follow-up. Continence and quality-of-life outcomes were recorded with the scoring system used in the respective study. When available, manometric parameters were also extracted.
In order to summarize patient and outcome data, descriptive statistics such as percentages, means, and total counts were used. As a result of the heterogeneity of patients and functional outcomes description, interpretation was limited to a pooled and selective descriptive analysis.
In those studies in which TEM or TAMIS was compared with other more invasive techniques, only data on minimally invasive approach were used.
The primary endpoint was continence; secondary endpoint included manometric results and QoL.
Statistical analysis
Data were extracted using the same scales and measurement units as from the original study. Due to the heterogeneity of the data, it was not possible to perform any pooled analysis or meta-analysis.
Results
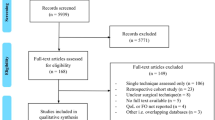
The PRISMA flowchart is shown in Fig. 1. After duplicate removal, a total of 1174 studies were identified. After screening for title and abstract, 105 studies were reviewed by full-text assessment, and 78 studies were excluded after not meeting inclusion criteria. The reasons for exclusion were as follows: 42 of them did not include preoperative data; 13 of them were written in other languages than English; 10 of them were literature reviews; 4 articles were case reports not meeting inclusion criteria; in 4 cases, there existed other studies from the same cohort with longer follow-up or more participants; 2 studies included previous or simultaneous abdominal surgery when TEM/TAMIS was performed; in 1 study, the surgery performed was transanal resection; 1 article was a study presentation; and another one only described technical notes. Two studies which were not detected in the systematic search were manually included after cross-referencing. A total of 29 studies, comprising 1297 patients, were included for final analysis.
Eighteen studies reported on results after TEM, and 4 studies after TAMIS. One study compared results between TEM and total mesorectal excision (TME), one between TEM and endoscopic mucosal resection (EMR), and one compared primary versus repeated TEM. Four studies included patients who underwent neoadjuvant therapy before transanal resection. Of them, one study described results of chemoradiotherapy (CRT) before TEM, and other before TAMIS. One study focused on patients who underwent short-course radiotherapy (SCRT) before TEM. Another study included two groups of patients comparing TEM alone versus CRT before TEM. No studies of TEO approach were retrieved.
Continence
Of the included articles, 23 (79%) reported on pre- and postoperative continence outcomes which are expressed in Table 2. Of them, 18 studies evaluated changes after TEM and 5 after TAMIS, with a mean follow-up of 15.9 months. A variety of continence assessment tools were used. Ten studies reported results using the Wexner score, seven used the Fecal Incontinence Severity Index (FISI), one study the colorectal functional outcome (COREFO) questionnaire, one study used both Wexner and COREFO scores, one study an individualized interview, and the remaining studies used the Kirwan-Fazio scale, the Williams score, and the Pescatori scale respectively.
TEM studies
Six studies reported on the outcomes using the Wexner score. Two studies found an increase in Wexner scores after surgery (worse continence) [17, 18], two studies found no changes in pre- and postoperative values [19, 20], and one found a decrease in the Wexner score (better continence) [21]. The sixth manuscript compared the results after a first versus repeated TEM, and found a worsening of continence after repeated TEM only, whereas no differences were noted between preoperative and postoperative functions after primary TEM [22].
Three studies described an improvement or worsening of continence after assessment with the Wexner score. One study reported a 21% incidence of de novo incontinence [23], and another one found an increase of 7% in incontinence after surgery in patients who underwent CRT + TEM [24]. The third study compared patients undergoing TEM alone and after CRT, and found that the latter had better continence in terms of soiling and urgency compared with TEM alone [25].
Five studies assessed patients using the FISI scale. Of them, three studies showed a decrease in FISI values (incontinence improvement) [26,27,28], whereas two studies did not show any changes [13, 29].
The study using an individualized interview found worsening in flatus and liquid stool incontinence, with no changes in gas incontinence after TEM [30]. After using the Kirwan-Fazio scale, Wang et al. [14] reported that 86.7% of patients showed no incontinence and 13.3% had flatus incontinence after TEM, while almost 45% of patients had any kind of incontinence preoperatively. The two studies that used the Williams score and the Pescatori scale showed no changes in continence after TEM [31, 32].
TAMIS studies
Among the five included studies, two used the Wexner scale and showed no changes in continence after surgery [33, 34]. Three studies used the FISI scale. Of them, two studies showed continence improvement by decreased FISI values [35, 36], while the other, which included patients who underwent CRT before TAMIS, found worse postoperative FISI values (continence impairment) [37].
Quality of life
Ten studies (34%) analyzed QoL changes as reflected in Table 3. Of them, nine involved TEM technique and one TAMIS, with a mean follow-up of 19 months. Six studies used the Fecal Incontinence Quality of Life (FIQL) index, six studies used the European Organization for Research and Treatment of Cancer (EORTC) C-30, one study the EORTC C-29, four studies the EORTC C-38, and three the EuroQol-5D scale. Half of the retrieved articles reported improvement in QoL [20, 27, 28, 36, 38], four remained comparable with preoperative values [13, 24, 26, 39], and only one study had worsening in some QoL components [40].
The first study analyzing QoL was published by Cataldo et al. [13], who used the FIQL in patients undergoing TEM. They showed minimal deterioration of the four components of the index (lifestyle, coping, depression, and embarrassment) with no statistical differences. On the other hand, three of the studies using the FIQL index before and after TEM showed statistical improvement in some components of the scale. “Lifestyle” score was increased in two studies [20, 27], “coping” in one [28], “depression” in two studies [20, 28], and “embarrassment” in three studies [20, 27, 28]. Arezzo et al. [40] reported impairment in all components of the FIQL index in patients who underwent TEM after neoadjuvant therapy, but this was not statistically significant. Only Verseveld et al. [36] focused on QoL after TAMIS, and found an improvement in “coping” and “embarrassment.”
The EORTC C-30 was used in six studies on TEM. Three studies concluded that all domains of the scale were comparable with preoperative values [20, 26, 39]. Another study comparing TEM with TME showed that patients who underwent TEM experienced an improvement in “insomnia,” “global health status,” and “physical and emotional functioning roles” [38]. Planting et al. [28] found improvement in “diarrhea” after TEM. However, Arezzo et al. [40] assessed ten patients who underwent TEM after SCRT, and found that the total EORTC C-30 score decreased in 12 items after surgery compared with preoperative values, which translates to QoL worsening.
Hompes et al. [26] also used the EORTC C-29 for QoL assessment reporting no differences in any domains to preoperative values.
Four studies included the EORTC C-38 test. Allaix et al. [20] showed that patients who underwent TEM had improved “body image,” “defecation problems,” and “future perspectives.” All other studies reported no changes in all domains, as compared with preoperative values [28, 38, 39]. The EuroQol-5D, an easier scale to assess QoL, was used in two TEM and one TAMIS studies. All of them showed improvement of the score [20, 27, 36].
Manometric outcomes
A total of fifteen studies (51%) investigated manometric variables pre- and postoperatively. All but one studies focused on patients who underwent TEM. Results are reported in Table 4. The most commonly investigated variables included anal resting pressure (ARP) and squeeze pressure (SP), followed by the presence of rectoanal inhibitory reflex (RAIR), rectal compliance, and rectal sensitivity.
Eight studies on TEM found a significant ARP decrease after surgery [14, 17, 18, 21, 30, 32, 42, 43], while three other showed no difference in ARP [19, 23, 29]. Biviano et al. [25] found only significant decrease in ARP in patients who had undergone CRT before TEM. Only three studies showed a decrease in SP [18, 32, 43]. One study found decreased rectal compliance and rectal sensitivity [30] after TEM. RAIR tended to decrease after TEM [23, 30, 42], and one study showed no changes after surgery [29].
The only study analyzing manometric outcomes after TAMIS showed significant decrease of APR and SP [33].
Discussion
There is great variability in the reporting of functional and QoL outcomes after TEM and TAMIS in available studies. The current systematic review with almost 1300 patients included found that most studies reported some deterioration in manometric scores after both TEM and TAMIS, and some suggested concerning worsening in function, at least in some items of the used scores, including de novo incontinence in some patients. However, QoL does not seem to be significantly affected after the procedures.
The implementation of TEM and TAMIS has radically changed the surgical approach to rectal polyps and early rectal cancer, since the rectal wall is partially excised and pelvic nerve injuries are potentially avoided. Many studies have analyzed the oncological outcomes associated with organ preservation techniques, but data on function and QoL results are scarce and controversial. Since different platforms are used for TEM and TAMIS, results of the two techniques are herewith considered in details separately, when possible.
Continence assessment remains difficult in these patients, irrespective of the transanal approach used. Even before surgery, many aged patients already refer incontinence much before rectal tumor diagnosis. The presence of a rectal tumor, especially in the lower third of the rectum, also makes fecal incontinence plausible due to decreased rectal wall elasticity, sphincter reflexes impairment, and mucus or blood discharge [4, 21, 30]. In fact, after tumor resection, continence was recovered or improved in many studies with both TEM [21, 26,27,28] and TAMIS [35, 36]. However, de novo or worsening of fecal incontinence was also reported in several studies assessing TEM included in this review [17, 18, 23, 30]. This could be explained by sphincter damage caused by anal dilation during the surgery with the rigid TEM rectoscopes or platforms that are 4 cm wide [19]. Moreover, scar formation after partial rectal wall resection reduces rectal compliance, which might also result in later onset of fecal incontinence and urgency [20]. Of note, some authors reported that postoperative incontinence after TEM was transient in many patients and improved at initial follow-up [19, 20, 25, 26]. This improvement may be explained by rectal scar healing and anal tone recovery after surgery.
In order to detect those patients who might develop fecal incontinence after TEM, Mora Lopez et al. [18] found that only closer distance to the anal verge seemed to affect continence. Other reported risk factors for fecal incontinence included male gender, age at surgery, surgical time, extended resection, and full-thickness resection [17, 23, 24]. Khoury et al. [22] found that continence was only affected after repeated TEM, which might suggest that multiple traumas on the anal sphincter complex may be responsible for incontinence.
There were two studies which included patients who underwent chemoradiotherapy before TEM [24, 25], and one before TAMIS [37]. It has been postulated that radiotherapy compromises muscle and nerve fiber integrity and reduces wall elasticity often leading to fecal incontinence, tenesmus, and urgency [44, 45]. This was reported by Ghiselli et al. (TEM) [24] and Clermonts et al. (TAMIS) [37]. However, Biviano and colleagues [25] reported a decrease in soiling and the same rate in urgency after CRT + TEM, while patients without CRT showed worsening in flatus incontinence, soiling, and urgency. Therefore, the role of radiotherapy in continence impairment for patients undergoing TEM or TAMIS is difficult to interpret.
Only ten studies evaluated QoL changes after transanal endoscopic surgery (nine after TEM and one after TAMIS) (Table 3). Of them, five studies used the FIQL, which is a specific psychometric tool to assess the quality of life in incontinent patients in the domains of lifestyle, coping, depression, and embarrassment [46]. After TEM, all of these domains improved in the reported results, especially for embarrassment [20, 27, 28]; the same finding was observed after TAMIS [36]. This is of vital importance since rectal tumors induce awareness of continence issues and its subsequent changes in daily activities. Tumor excision not only may improve symptoms but also decreases psychological disorders induced by the fact of having a tumor, and even in those studies with a long follow-up, this improvement is maintained over time [20]. The fact that these techniques preserve the rectum without the implantation of a stoma justifies the high rates of quality-of-life scores in these patients.
Other studies used the EORTC questionnaires, which evaluate areas common to different tumor sites and treatments. After systematic analysis, almost all studies except for one showed that—with TEM—all domains were comparable with preoperative values, or improvement in certain domains such as defecation problems [20], or physical and emotional functioning [38]. Since these questionnaires do not focus on specific issues related to rectal tumors, it is most likely that patients do not report changes or that these changes are reported within the first postoperative months [38]. One study, however, did show QoL worsening using these general questionnaires [40]. This study included patients who underwent SCRT and TEM, which induced serious wound healing complications that might have impaired QoL (e.g., enterocutaneous fistula). The authors hypothesized that SCRT-TEM could provide similar oncological results to radical surgery with better QoL outcomes, but due to the incidence of serious postoperative complications after this approach, they concluded that QoL in these irradiated patients is probably equivalent or worse compared with radical surgery [40].
Patients who underwent manometry before surgery showed lower threshold volume, maximal tolerable volume, and rectal compliance due to the presence of the rectal tumor [23]. After surgery, the majority of studies reported a decrease in ARP and SP and in the presence of RAIR. This could be explained by the resection and scar formation in the rectum, especially in cases of extended and full-thickness resection, which induces a decrease in the rectal wall elasticity and cessation of local reflexes such as RAIR. These changes in anal reflexes might be responsible for sphincter function inhibition, disturbed defecatory coordination, and fecal incontinence or urgency onset both with TEM [23] and with TAMIS [33, 37]. As previously discussed, induced trauma in anal sphincters after platform insertion might also induce striated and smooth muscle sphincter lesions and fibrosis, which can provoke worsening in manometric parameters.
However, all these changes are mainly detected within the first postoperative months, and seem to recover at 1 year after surgery; hence, they might not be outline clinically relevant to the patients [18, 21]. As a matter of fact, it is hard to correlate manometric changes with continence assessment. The current systematic review included studies with continence deterioration and decreased pressures [17, 18, 30], continence improvement and decreased pressures [14, 21], and others in which there were neither changes in continence nor manometric pressures [19, 20, 29, 33] after TEM and TAMIS.
Only one study analyzed manometric outcomes in patients who underwent preoperative radiotherapy before TEM compared with those who underwent surgery alone [25]. Even though there were worse intermediate results in both groups, only patients who underwent surgery alone recovered the reported reduction in anal canal pressure, rectal compliance, and sensitivity at 1 year of follow-up. The authors suggest that TEM with CRT could cause more damage due to changes in the nerve fibers of the inner walls of the anal canal and/or the muscular structure of the sphincter which might impair tissue remodeling.
Study limitations
The main limitation of this review is that retrieved data from the studies were heterogeneous. Not only outcome data were expressed in different formats (mean scores, percentage of patients affected, figures), but also continence and QoL assessment were evaluated in different scores and follow-up sequences. This made it impossible to perform a complete standardized comparison or meta-analysis. Another bias in assessing continence changes in this study was the lack of information of previous medical history which could have been responsible for incontinence, such as obstetric trauma.
Another limitation to account for is that anorectal manometry normal values are influenced by technique, age, and gender. Therefore, the reported outcomes are difficult to interpret and extrapolate, especially in the presence of a rectal tumor. Also, there were no strict definitions of incontinence in any of the studies.
This review focused on functional and quality-of-life outcomes after TEM and TAMIS. The aim of these techniques is similar, but the materials used in them differ. TAMIS may have a less harmful influence on fecal continence since a flexible port is used instead of a rigid rectoscope as in TEM, but data is scarce and inconclusive.
For all the above-reported limitations, potential biases could not be removed; therefore, caution is warranted when drawing conclusions on function and QoL after TEM and TAMIS.
However, the current study has strength. The review methodology was rigorous and followed the PRISMA recommendations; studies were thoroughly assessed by two independent screeners, and despite the different scores and scales used in the studies, the clinical relevance of the findings was taken into account. Several interesting results were observed. The possibility of worsened function after TEM and TAMIS should not be underestimated, even if the reasons underlying this are yet to be completely understood and are likely to be multifactorial. These techniques do not seem to affect continence by themselves, except in minor cases. Also, manometric findings did not always correlate with function. Of note, QoL does seem to be stable or improved after TEM or TAMIS. However, the perception of having being cured (avoiding a stoma) could be responsible for the improved QoL scores, and longer follow-ups or more specific QoL tools might capture more issues. All these aspects need to be addressed in future studies and should be discussed with patients at the time of counseling. There is the need to homogenize or standardize functional and manometric outcomes assessment after TEM or TAMIS.
References
Peeters KCMJ, van de Velde CJH, Leer JWH, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA (2005) Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol Off J Am Soc Clin Oncol 23:6199–6206. https://doi.org/10.1200/JCO.2005.14.779
Dahlberg M, Glimelius B, Graf W, Påhlman L (1998) Preoperative irradiation affects functional results after surgery for rectal cancer: results from a randomized study. Dis Colon Rectum 41:543–549 discussion 549-551
Pollack J, Holm T, Cedermark B et al (2006) Long-term effect of preoperative radiation therapy on anorectal function. Dis Colon Rectum 49:345–352. https://doi.org/10.1007/s10350-005-0296-1
Nesbakken A, Nygaard K, Bull-Njaa T et al (2000) Bladder and sexual dysfunction after mesorectal excision for rectal cancer. Br J Surg 87:206–210. https://doi.org/10.1046/j.1365-2168.2000.01357.x
Havenga K, Enker WE, McDermott K, Cohen AM, Minsky BD, Guillem J (1996) Male and female sexual and urinary function after total mesorectal excision with autonomic nerve preservation for carcinoma of the rectum. J Am Coll Surg 182:495–502
Buess G, Theiss R, Hutterer F, Pichlmaier H, Pelz C, Holfeld T, Said S, Isselhard W (1983) Transanal endoscopic surgery of the rectum - testing a new method in animal experiments. Leber Magen Darm 13:73–77
Atallah S, Albert M, Larach S (2010) Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 24:2200–2205. https://doi.org/10.1007/s00464-010-0927-z
Moore JS, Cataldo PA, Osler T, Hyman NH (2008) Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum 51:1026–1030; discussion 1030-1031. https://doi.org/10.1007/s10350-008-9337-x
Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW (2013) Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum 56:301–307. https://doi.org/10.1097/DCR.0b013e31827ca313
deBeche-Adams T, Nassif G (2015) Transanal minimally invasive surgery. Clin Colon Rectal Surg 28:176–180. https://doi.org/10.1055/s-0035-1555008
Serra-Aracil X, Pericay C, Golda T, Mora L, Targarona E, Delgado S, Reina A, Vallribera F, Enriquez-Navascues JM, Serra-Pla S, Garcia-Pacheco JC, TAU-TEM study group (2018) Non-inferiority multicenter prospective randomized controlled study of rectal cancer T2-T3s (superficial) N0, M0 undergoing neoadjuvant treatment and local excision (TEM) vs total mesorectal excision (TME). Int J Color Dis 33:241–249. https://doi.org/10.1007/s00384-017-2942-1
Pellino G, Warusavitarne J (2017) Medium-term adoption trends for laparoscopic, robotic and transanal total mesorectal excision (TaTME) techniques. Tech Coloproctol 21:911–913. https://doi.org/10.1007/s10151-017-1719-4
Cataldo PA, O’Brien S, Osler T (2005) Transanal endoscopic microsurgery: a prospective evaluation of functional results. Dis Colon Rectum 48:1366–1371. https://doi.org/10.1007/s10350-005-0031-y
Wang H-S, Lin J-K, Yang S-H, Jiang JK, Chen WS, Lin TC (2003) Prospective study of the functional results of transanal endoscopic microsurgery. Hepatogastroenterology 50:1376–1380
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(264–269):W64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Deeks JJ, Dinnes J, D’Amico R et al (2003) Evaluating non-randomised intervention studies. Health Technol Assess Winch Engl 7:iii–iix 1–173
Kennedy ML, Lubowski DZ, King DW (2002) Transanal endoscopic microsurgery excision: is anorectal function compromised? Dis Colon Rectum 45:601–604
Mora López L, Serra Aracil X, Hermoso Bosch J et al (2015) Study of anorectal function after transanal endoscopic surgery. Int J Surg Lond Engl 13:142–147. https://doi.org/10.1016/j.ijsu.2014.11.021
Jin Z, Yin L, Xue L, Lin M, Zheng Q (2010) Anorectal functional results after transanal endoscopic microsurgery in benign and early malignant tumors. World J Surg 34:1128–1132. https://doi.org/10.1007/s00268-010-0475-7
Allaix ME, Rebecchi F, Giaccone C, Mistrangelo M, Morino M (2011) Long-term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg 98:1635–1643. https://doi.org/10.1002/bjs.7584
Barendse RM, Oors JM, de Graaf EJR et al (2013) The effect of endoscopic mucosal resection and transanal endoscopic microsurgery on anorectal function. Color Dis 15:e534–e541. https://doi.org/10.1111/codi.12311
Khoury W, Gilshtein H, Nordkin D, Kluger Y, Duek SD (2013) Repeated transanal endoscopic microsurgery is feasible and safe. J Laparoendosc Adv Surg Tech A 23:216–219. https://doi.org/10.1089/lap.2012.0394
Herman RM, Richter P, Walega P, Popiela T (2001) Anorectal sphincter function and rectal barostat study in patients following transanal endoscopic microsurgery. Int J Color Dis 16:370–376
Ghiselli R, Ortenzi M, Cardinali L, Skrami E, Gesuita R, Guerrieri M (2017) Functional outcomes after TEM in patients with complete clinical response after neoadjuvant chemoradiotherapy. Surg Endosc 31:2997–3003. https://doi.org/10.1007/s00464-016-5321-z
Biviano I, Balla A, Badiali D et al (2017) Anal function after endoluminal locoregional resection by transanal endoscopic microsurgery and radiotherapy for rectal cancer. Color Dis 19:O177–O185. https://doi.org/10.1111/codi.13656
Hompes R, Ashraf SQ, Gosselink MP et al (2015) Evaluation of quality of life and function at 1 year after transanal endoscopic microsurgery. Color Dis 17:O54–O61. https://doi.org/10.1111/codi.12858
Doornebosch PG, Gosselink MP, Neijenhuis PA, Schouten WR, Tollenaar RA, de Graaf EJ (2008) Impact of transanal endoscopic microsurgery on functional outcome and quality of life. Int J Color Dis 23:709–713. https://doi.org/10.1007/s00384-008-0442-z
Planting A, Phang PT, Raval MJ, Brown CJ (2013) Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg J Can Chir 56:243–248. https://doi.org/10.1503/cjs.028411
Wałęga P, Kenig J, Richter P (2014) Transanal endoscopic microsurgery combined with endoscopic posterior mesorectum resection in the treatment of patients with T1 rectal cancer - 3-year results. Wideochirurgia Inne Tech Maloinwazyjne Videosurgery Miniinvasive Tech 9:40–45. https://doi.org/10.5114/wiitm.2014.40384
Kreis ME, Jehle EC, Haug V, Manncke K, Buess GF, Becker HD, Starlinger MJ (1996) Functional results after transanal endoscopic microsurgery. Dis Colon Rectum 39:1116–1121
Meng WCS, Lau PYY, Yip AWC (2004) Treatment of early rectal tumours by transanal endoscopic microsurgery in Hong Kong: prospective study. Hong Kong Med J Xianggang Yi Xue Za Zhi 10:239–243
Gracia Solanas JA, Ramírez Rodríguez JM, Aguilella Diago V et al (2006) A prospective study about functional and anatomic consequences of transanal endoscopic microsurgery. Rev Espanola Enfermedades Dig Organo Of Soc Espanola Patol Dig 98:234–240
Karakayali FY, Tezcaner T, Moray G (2015) Anorectal function and outcomes after transanal minimally invasive surgery for rectal tumors. J Minimal Access Surg 11:257–262. https://doi.org/10.4103/0972-9941.152094
García-Flórez LJ, Otero-Díez JL, Encinas-Muñiz AI, Sánchez-Domínguez L (2017) Indications and outcomes from 32 consecutive patients for the treatment of rectal lesions by transanal minimally invasive surgery. Surg Innov 24:336–342. https://doi.org/10.1177/1553350617700803
Schiphorst AHW, Langenhoff BS, Maring J, Pronk A, Zimmerman DD (2014) Transanal minimally invasive surgery: initial experience and short-term functional results. Dis Colon Rectum 57:927–932. https://doi.org/10.1097/DCR.0000000000000170
Verseveld M, Barendse RM, Gosselink MP et al (2016) Transanal minimally invasive surgery: impact on quality of life and functional outcome. Surg Endosc 30:1184–1187. https://doi.org/10.1007/s00464-015-4326-3
Clermonts SHEM, van Loon YT, Schiphorst AHW, Wasowicz DK, Zimmerman DDE (2017) Transanal minimally invasive surgery for rectal polyps and selected malignant tumors: caution concerning intermediate-term functional results. Int J Color Dis 32:1677–1685. https://doi.org/10.1007/s00384-017-2893-6
Lezoche E, Paganini AM, Fabiani B, Balla A, Vestri A, Pescatori L, Scoglio D, D'Ambrosio G, Lezoche G (2014) Quality-of-life impairment after endoluminal locoregional resection and laparoscopic total mesorectal excision. Surg Endosc 28:227–234. https://doi.org/10.1007/s00464-013-3166-2
D’Ambrosio G, Balla A, Mattei F et al (2015) Quality of life after endoluminal loco-regional resection (ELRR) by transanal endoscopic microsurgery (TEM). Ann Ital Chir 86:56–60
Arezzo A, Arolfo S, Allaix ME et al (2015) Results of neoadjuvant short-course radiation therapy followed by transanal endoscopic microsurgery for t1-t2 n0 extraperitoneal rectal cancer. Int J Radiat Oncol Biol Phys 92:299–306. https://doi.org/10.1016/j.ijrobp.2015.01.024
Hemingway D, Flett M, McKee F, Finlay IG (1996) Sphincter function after transanal endoscopic microsurgical excision of rectal tumours. British Journal of Surgery 83: 51–52
Banerjee AK, Jehle EC, Kreis ME et al (1996) Prospective study of the proctographic and functional consequences of transanal endoscopic microsurgery. Br J Surg 83:211–213
Léonard D, Colin J-F, Remue C, Jamart J, Kartheuser A (2012) Transanal endoscopic microsurgery: long-term experience, indication expansion, and technical improvements. Surg Endosc 26:312–322. https://doi.org/10.1007/s00464-011-1869-9
Da Silva GM, Berho M, Wexner SD et al (2003) Histologic analysis of the irradiated anal sphincter. Dis Colon Rectum 46:1492–1497. https://doi.org/10.1097/01.DCR.0000093642.89267.67
Yarnold J, Brotons M-CV (2010) Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol J Eur Soc Ther Radiol Oncol 97:149–161. https://doi.org/10.1016/j.radonc.2010.09.002
Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, Wexner SD, Bliss D, Lowry AC (1999) Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum 42:1525–1532
Acknowledgments
The authors would like to thank Dr. Dimelza Osorio Sánchez for her assistance during the search process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix



Rights and permissions
About this article
Cite this article
Marinello, F.G., Curell, A., Tapiolas, I. et al. Systematic review of functional outcomes and quality of life after transanal endoscopic microsurgery and transanal minimally invasive surgery: a word of caution. Int J Colorectal Dis 35, 51–67 (2020). https://doi.org/10.1007/s00384-019-03439-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03439-3