Abstract
Background
Crohn’s disease (CD) patients after ileal anal pouch anastomosis (IPAA) are subject to CD recurrence, septic complications, and pouch failure. The aim of this study was to compare long-term outcomes of index and redo IPAA for CD.
Methods
Patients who underwent index and redo IPAA with a diagnosis of CD colitis were identified from a prospectively maintained IPAA database. Charts were reviewed to determine complications and pouch failure rates after index and redo IPAA. Long-term pouch survival and quality of life (QoL) were compared between index and redo IPAAs.
Results
There were 305 patients, 253 with an index IPAA and 52 having a redo IPAA. Their median ages were 33 years (index IPAA) and 32 years (redo IPAA) (p = 0.91); there were 47% and 53% men in each group, respectively (p = 0.54). Pouch salvage with redo IPAA was possible in 75% of redo pouches. Biologic agents were given in 8% of index IPAA and 34% redo IPAA patients (p < 0.01). Cumulative Kaplan Meier 5-year pouch survival was 80% vs. 60% in index and redo IPAA (p < 0.0001), at 10 years 74% vs. 38%, respectively (p < 0.0001). When queried, 78% who underwent redo pouch surgery would have it again and 86% would recommend this surgery to others.
Conclusion
IPAA can be offered to selected patients with isolated colonic CD. Failure in this group of patients is related mainly to recurrent CD, not surgical complications. Redo IPAA is a realistic option for salvage in certain patients with failed index IPAA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ileal pouch anal anastomosis (IPAA) has become the gold standard procedure for patients with ulcerative colitis (UC) and familial adenomatous polyposis (FAP). In selected patients that develop pouch failure related to fistula, pouch malposition or other selected conditions, a redo pouch may be offered with acceptable results [1].
Typically, patients with Crohn’s disease (CD) are not considered for IPAA [2]. However, an ileal pouch anal anastomosis (IPAA) can be safe and feasible in highly selected patients with CD limited to the large intestine only [3,4,5,6]. Our institution found that patients who were highly motivated to avoid a permanent stoma with known preoperative CD had a 10-year pouch retention rate of 71%, median of 7 bowel movements daily, and similar functional results compared to our patients with UC and FAP [5].
There are some patients with CD who develop IPAA problems where a redo pouch would be feasible [6, 7]. There is little data to guide counseling decisions regarding results of redo IPAA for CD patients. The aim of this study was to compare the outcomes of index and redo IPAA for patients with CD and estimate if redo IPAA is a safe and feasible option for CD patients.
Methods
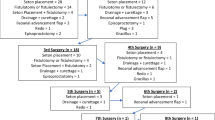
After Institutional Review Board Committee approval, a prospectively maintained pouch database was queried. All patients who underwent IPAA surgery (index or redo) in the Department of Colon and Rectal Surgery (Cleveland, Ohio) with a diagnosis of CD colitis between 1983 and 2015 were included in this study. Patients were divided into 2 groups: index and redo IPAA (redo IPAA was through transabdominal access). Patients with FAP and cancer were excluded.
CD diagnosis was classified into intentional (clinical symptoms preoperatively), incidental (clinical presentation suspecting UC or IC undergoing IPAA with diagnosis converting to CD on the basis of postoperative histopathology of colon and rectum), and delayed (clinical symptoms and endoscopic findings which develop over time after resection of colon and rectum and creation of index IPAA for presumable diagnosis of UC) [8,9,10]. Delayed CD diagnosis was not made within first 3 months after ileostomy reversal in order to avoid labeling patients with CD who instead developed postoperative technical complications. Thus, patients were diagnosed with CD either intentionally, incidentally or had a conversion of their preoperative diagnosis of UC or IC to CD after construction of index or redo IPAA.
Baseline demographic data was obtained retrospectively from medical records including age at CD diagnosis, age at index IPAA, time to redo IPAA after index IPAA, preoperative body mass index (BMI), American Society of Anesthesiology (ASA) class, hemoglobin levels, preoperative diagnosis before index and redo IPAA, conversion of clinical diagnosis over time, and perioperative medical treatment. Intraoperative details analyzed included index pouch configuration (J/S), anastomosis type (handsewn/stapled), estimated blood loss, duration of surgery, and length of hospital stay.
The primary outcome was pouch failure in index and redo IPAA. Secondary outcomes were pouch function and quality of life (QoL) after index and redo IPAAs. Pouch failure was defined as either pouch excision with end ileostomy or permanent pouch diversion. Pouch retention rates were analyzed by cumulative Kaplan Meier pouch survival analysis at 1-, 3-, 5-, and 10-year follow-up.
Criteria for redo IPAA in CD
At the time of index pouch, there was minimal perianal disease and no small bowel involvement. Multiple perianal abscesses and fistulas or extensive perianal disease, were considered a contraindication for redo procedure. Redo IPAA was offered to patients with the diagnosis of CD colitis, who developed isolated pouch-targeted/vaginal fistulas, anastomotic leaks, pelvic sepsis, and pouch dysfunction, which were anticipated to be related to technical issue at the primary pouch formation. Isolated anal fistulas located in the anal transition zone (ATZ) were thought to be most likely related to CD and were considered for redo pouch if there was a single tract, there was no evidence of sepsis, and there was minimal inflammation at the ATZ. A redo pouch was considered feasible if on pouchoscopy pelvic pouch mucosa was unremarkable and CD of the pouch, active small bowel as well as extensive perianal disease were excluded. Patients were not considered candidates for redo IPAA if CD recurred in the prepouch ileum or the body of the pouch.
Technique
Redo IPAA was defined as anastomotic disconnection with IPAA reconstruction with either the revised/repaired original pouch or after creation of new ileal pouch. All redo IPAAs were performed in three stages including initial diverting loop ileostomy, redo procedure, and ileostomy closure.
We compared the operative technique of index and redo IPAA in CD such as creation of new or salvage of old pouch, rates of mucosectomy and stapled redo IPAAs, new pouch configuration, conversion of index pouch configuration from S to J and vice versa, and duration of surgery.
Postoperative complications were compared between the groups including anastomotic leak, pelvic sepsis, fistulas, stricture, pouchitis, and wound infection. We also studied median time to fistula development and its location. Patients had multiple complications during follow-up; thus, summation of each complication exceeds total overall number.
The Cleveland Clinic Pelvic Pouch Questionnaire (CGQoL), a self-administered, validated questionnaire, was used to assess function and QoL after IPAA at the most recent follow-up [11]. Questionnaires included questions to address bowel frequency (number of bowel movements per 24 h), stool seepage (soiling during the day or night), dietary, social, work, and sexual, restrictions [11, 12].
Patients were followed with pouch endoscopy every 1–2 years. During endoscopy, biopsies were obtained of the pouch body, afferent limb, and ATZ. After IPAA, pouch dysfunction was evaluated with an exam under anesthesia, pouchoscopy, radiologic testing (gastrograffin enemas, CT enterography, and magnetic resonance imaging), and histopathologic analysis. All information was compiled to elicit reasons for IPAA failure.
Statistical methods
The time periods were compared with respect to categorical variables using chi-square or Fisher’s exact tests. Comparisons with respect to quantitative variables were performed using the Kruskal-Wallis test, of which the Wilcoxon rank sum test was a subset for pairwise group comparisons. The likelihood of follow-up complications, including pouch failure, was estimated using the Kaplan-Meier method, and comparisons among time periods and other univariable analyses for complications were assessed using log rank tests. Quality of life endpoints was analyzed with respect to most recent measurements, but also at 1-, 5-, and 10-year time points. Multivariable Cox regression models were constructed for pouch failure for the entire analysis population, and also for the individual time periods. Analyses were performed using R version 2.15.1 (www.r-project.org).
Results
From 1983 to 2015, out of 4592 ileal pouches identified in the database a total of 305 patients underwent IPAA for CD colitis: 253 patients had an index IPAA and 52—redo IPAA. The redo IPAA group consisted of patients who underwent index IPAA at our institution and required revision/redo, as well as patients referred from outside units for redo pouch surgery. Groups were similar in terms of age, gender, BMI, and preoperative disease duration. At the time of index and redo IPAA, median ages were 33 and 32 years (p = 0.91), respectively (Table 1). The median age at index IPAA surgery for those who had redo IPAA was 28 (11–51) years. The median time to redo IPAA after index IPAA was 6 (0.4–24.3) years. Median follow-up was 8.7 (3.7–30.4) in index and 2.2 (0.5–24) years in the redo group, respectively (p < 0.01).
A total of 29 (56%) patients in the redo IPAA compared to 63 (25%) patients in index IPAA (p < 0.0001) group underwent intentional IPAA for CD. Delayed CD was established in 23 (44%) in the redo cohort compared to 58 (23%) patients in the index IPAA cohort (p < 0.001). Incidental CD was noted in 132 (52%) patients in index IPAA group.
Reasons for redo IPAA were fistulas—26 (53%)(pouch-vaginal fistula—17, pouch-perineal fistula—5, abdominal wall fistulas—4), anastomotic leak—9 (17%), pelvic abscess—4 (7.5%), pouch dysfunction—6 (9.4%), stricture—7 (13.2%). Out of these, 14 (27%) patients had a combination of problems that led to redo pouch.
Patients from the redo group underwent treatment with biologic medications more frequently than in index IPAA (18 (34%) vs. 21 (8%), p < 0.0001)). In the redo group, 7 patients received biologics before redo IPAA for presumed CD relapse, aiming to salvage index pouch, while the rest were treated after redo IPAA. In the index group, biologics were given before pouch creation.
Redo IPAA was associated with longer operative time and higher estimated blood loss when compared to index IPAA patients. In index IPAA, a two-staged IPAA was performed in 137 (54%) and three-staged in 116 (46%) cases. J pouch configuration was utilized in over 80% of patients in index and redo pouch surgery. In the redo group, the original pouch was repaired and preserved in 40 (75%) patients, while the rest 12 (25%) had excision of an old and creation of new ileal pouch. Stapled IPAA was utilized in 164 (74%) within the index IPAA cohort and in 14 (33%) patients in the redo cohort, while mucosectomy in 66 (26%) and 36 (67%) in the index and redo groups, respectively (p< 0.001) (Table 1).
Rates of anastomotic separation, pelvic sepsis, fistulas, anastomotic strictures, and pouchitis were comparable between groups (Table 2). Fistulas were the most prevalent complication after ileostomy reversal in both groups. The median time to development of fistula was 1.5 (0.2–1.8) and 1.3 (0.5–2) years in the index IPAA and redo groups, respectively (p = 0.59). There were no differences noted in fistula locations in relation to ileal pouch between the index and redo pouch groups: cryptoglandular—46 (18%) vs. 2 (4%), p = 0.13; anastomosis site—15 (6%) vs. 3 (6%), p = 0.93; pouch body—7 (3%) vs.1 (2%), p = 0.71; presacral fistulas—9 (3%) vs. 0, p = 0.16, pouch-vaginal—6 (2%) vs. 4 (7%), p = 0.05.
As expected, pouch failure rate was higher after redo than index IPAA: 24 (45%) vs. 72 (28%), p < 0.001. The cumulative 5-year pouch survival after index and redo IPAA were 79% vs. 60%, p = 0.001, at 10 years—74% vs. 38%, p = 0.001, respectively (Table 2, Fig. 1). Both index and redo IPAA failures were related to CD recurrence in 31 (43%) vs. 15 (62%), p = 0.09. Other reasons for pouch failure were anastomotic separation, pelvic sepsis, recurrent IPAA strictures, pouchitis, and pouch dysfunction (Table 2), which were similar between the groups. Patients which developed pouch-targeted fistulas had significantly lower rates of redo IPAA retention vs. index IPAA at 5 and 10 years (40% (14%–65%) vs. 72% (48%–87%), at 5 years after redo and index IPAA and 13% (8%–43%) vs. 53% (27%–73%) at 10 years after redo and index IPAA).
Seepage rate was better in index compared to redo pouches (35% vs. 41%, p = 0.56), and similar during nighttime (43% vs. 46% in index and redo groups, p = 0.80) (Table 3). Stool frequency was similar between groups: 7.5 ± 4.4 in index and 6.1 ± 3.6 in redo groups (p = 0.10). The median overall CGQoL scores were 0.8 (0.6–1) and 0.7 (0.4–1) in the index and redo IPAA groups, respectively (p = 0.62). The vast majority of patients reported that they would be willing to undergo surgery again (220 (87%) vs. 40 (78%) in index vs. redo groups, p = 0.18). Patients rated their satisfaction with surgery after index and redo IPAA as 7 (3–9) and 6 (3–9) (p = 0.36).
Univariate logistic regression analysis identified fistula as a significant factor for pouch failure in the index and redo groups (Table 4). The stepwise Cox proportional hazard ratio analysis demonstrated redo surgery as significant factor leading pouch failure, as well as postoperatively developed pelvic sepsis, pouch-targeted fistula and younger age at surgery (Table 5). Subsequent models for index and redo IPAA demonstrated fistula, pelvic sepsis, and younger age at surgery to increase risk of pouch failure. Multivariate subanalysis did not identify statistically significant factors leading to redo pouch failure, probably due to low number of patients and pouch failure events (Table 5).
Discussion
Constructing a pelvic pouch for patients with CD would be considered a contraindication by the majority of surgeons. Even fewer surgeons would perform a redo pouch in a patient with CD. However, our group showed acceptable outcomes after IPAA when performed for preoperatively known CD [1,2,3,4].
In this study, we investigated if pouch failure after IPPA mandates a permanent ileostomy or if a redo pouch procedure is a safe and feasible alternative. In this patients’ cohort, pouch failure occurred in 72 (28%) cases after index pouch procedure and in 24 (45%) cases after redo pouch (p < 0.001) with median follow-up of 8 and 3 years, respectively. Although Kaplan-Meier 5- and 10-year pouch survival rates were significantly better in index compared to redo pouch: 79% vs. 60%, p = 0.001 and 74% vs. 38%, p = 0.001, this data supports the idea of having an opportunity to avoid a permanent ileostomy even if index pouch failed. Fazio et al. reported that 87% patients with CD during median follow-up of 84 months would retain functional ileal pouch [4]. Melton et al. reported that 71% patients with CD would retain functional ileal pouch at 10-year follow-up, and 85% if performed intentionally for preoperatively known CD, 87% if CD discovered incidentally based on pathology results, and 53% if CD diagnosed in delayed setting [3].
After both index and redo pouch surgeries, IPAA-related complications such as anastomosis dehiscence and pelvic sepsis were less prevalent than pouch-targeted fistulas, pouchitis, and strictures, which probably were related to CD relapse. Rates of postoperative complications in both index and redo groups correlate with previously reported postoperative morbidity rates by Melton et al. which were pouchitis (54%), fistulous disease (35%, including pouch-vaginal fistula (24%) and perianal fistula (25%)), and IPAA strictures (24%) [3].
Redo pouch surgery is associated with higher chances of pouch failure based on our multivariate analysis. Postoperative pelvic sepsis, pouch targeted fistula and younger age at surgery are significant factors associated with pouch failure among CD patients. In the literature, factors associated with IPAA failure in CD were delayed CD diagnosis, pouch-vaginal fistula, postoperative pelvic sepsis, active smoking, seropositive anti-Saccharomyces cerevisiae IgA (ASCA), family history of CD, and longer time of having IPAA, what corresponds with our results [8, 10, 13]. Patients with delayed CD diagnosis seem to be predisposed to later small bowel involvement, more severe ongoing disease, leading to CD of the ileal pouch [3]. In this series, neither intentional nor delayed CD diagnosis was significantly associated with redo pouch failure in stepwise regression analysis, what could be related to a low number of patients.
Patients in the redo group underwent treatment with biologic medications more frequently than in index IPAA patients (18 (34%) vs. 21 (8%), p < 0.0001)). Seven patients (11%) in the redo group received biologics before redo pouch aiming to salvage the pouch, but ultimately underwent redo IPAA. Eleven patients (19%) in the redo group received biologics therapy after redo surgery to salvage their redo pouches and 7 (13%) patients out of those failed redo pouch as well as ended with a permanent stoma. Our series lack statistical power to provide answers whether perioperative use of biologics might prolong time to redo pouch or salvage a failing ileal pouch. However, it remains unknown if CD pouch patients should be offered medical therapy in an effort to delay or avoid CD-related problems after recovery from surgery.
We did not identify statistical difference in QoL or pouch function after index and redo IPAAs, although there tended to be higher QoL restrictions and seepage rates in the redo group. In spite of higher pads usage, patients in both groups reported willingness to undergo the same surgery again, especially in redo group. Despite there was no difference in QoL scores between the groups, we recognize that patients who undergo redo pouch surgery with CD are highly motivated patients willing to undergo multiple reoperations in order to avoid end ileostomy.
Our results provide evidence which would be of benefit in preoperative counseling and assist to set realistic goals and expectations for patients with CD, in particular patients with CD colitis, who underwent IPAA and pouch failure occurred, who are willing to understand the risks of redo pouch procedure.
Patients with CD have acceptable similar rates of postoperative complications and functional outcomes after both index and redo IPAA, however with significantly higher risk of redo pouch failure within shorter period of time when compared to index IPAA. We strongly feel that patients’ selection based on a meticulous multidisciplinary preoperative evaluation is the key to success of IPAA for those with CD, and in particular for redo IPAA.
Limitations of this study are related to the retrospective design performed within a single institution. The study covers a very long period of time (1983–2015), which may have led to heterogeneity and consecutive bias. The volume of redo IPAA surgery is low, but is even lower for patients with CD diagnosis, and thus conclusions of this paper comes from a small number of patients. However, despite these limitations, our results suggest similar complications and pouch function after index and redo IPAAs, what suggests that redo IPAA is a safe option for selected patients with CD but with higher redo pouch failure rates on the long-term. Such good long-term results can only be achieved by a specialized interdisciplinary team of CD/IBD experts at a high-volume surgical center. Therefore, the external validity and generalizability of the presented results is highly limited.
Conclusions
In highly selected motivated patients with colorectal CD and no small bowel disease, a redo IPAA may be a feasible alternative to a pouch excision, with acceptable functional outcome comparable to index pouch procedure. Redo pouch failure rates are higher compared to index pouch; however, 60% of patients retain a functional redo pouch in situ within 5 years and almost 40% at 10 years.
References
Remzi FH, Aytac E, Ashburn J, Gu J, Hull TL, Dietz DW, Stocchi L, Church JM, Shen B (2015) Transabdominal redo ileal pouch surgery for failed restorative proctocolectomy: lessons learned over 500 patients. Ann Surg 262:675–682
Joyce MR, Fazio VW (2009) Can ileal pouch anal anastomosis be used in Crohn’s disease? Adv Surg 43:111–137
Melton GB, Fazio VW, Kiran RP, He J, Lavery IC, Shen B, Achkar JP, Church JM, Remzi FH (2008) Long-term outcomes with ileal pouch-anal anastomosis and Crohn’s disease: pouch retention and implications of delayed diagnosis. Ann Surg 248:608–616
Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, Manilich E, Shen B, Martin ST (2013) Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 257:679–685
Siassi M, Weiger A, Hohenberger W, Kessler H (2007) Changes in surgical therapy for Crohn’s disease over 33 years: a prospective longitudinal study. Int J Color Dis 22:319–324
Le Q, Melmed G, Dubinsky M et al (2013) Surgical outcome of ileal pouch-anal anastomosis when used intentionally for well-defined Crohn’s disease. Inflamm Bowel Dis 19:30–36
Garrett KA, Remzi FH, Kirat HT, Fazio VW, Shen B, Kiran RP (2009) Outcome of salvage surgery for ileal pouches referred with a diagnosis of Crohn’s disease. Dis Colon Rectum 52:1967–1974
Shen B, Patel S, Lian L (2010) Natural history of Crohn’s disease in patients who underwent intentional restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment Pharmacol Ther 31:745–753
Peyregne V, Francois Y, Gilly FN et al (2000) Outcome of ileal pouch after secondary diagnosis of Crohn’s disease. Int J Color Dis 15:49–53
Shen B, Fazio VW, Remzi FH, Lashner BA (2005) Clinical approach to diseases of ileal pouch-anal anastomosis. Am J Gastroenterol 100:2796–2807
Fazio VW, O'Riordain MG, Lavery IC et al (1999) Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg 230:575–584 discussion 584-6
Kiran RP, Delaney CP, Senagore AJ, O'Brien-Ermlich B, Mascha E, Thornton J, Fazio VW (2003) Prospective assessment of Cleveland Global Quality of Life (CGQL) as a novel marker of quality of life and disease activity in Crohn's disease. Am J Gastroenterol 98:1783–1789
Shen B, Fazio VW, Remzi FH et al (2006) Risk factors for clinical phenotypes of Crohn’s disease of the ileal pouch. Am J Gastroenterol 101:2760–2768.10
Author information
Authors and Affiliations
Contributions
All authors participated in conception and design of the study, acquisition of data, analysis and interpretation of data, drafting and revising the manuscript, and approval of the published version.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was Podium at the Meeting of American College of Surgeons, October 15–19, 2016, Washington, USA.
Rights and permissions
About this article
Cite this article
Lavryk, O.A., Hull, T.L. Comparison of long-term outcomes of primary and redo IPAA for patients with Crohn’s disease. Int J Colorectal Dis 34, 1945–1951 (2019). https://doi.org/10.1007/s00384-019-03411-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03411-1