Abstract
Purpose
Type 2 diabetes mellitus (diabetes) is a common comorbid condition among older adult colorectal cancer (CRC) patients, yet its effects on CRC mortality have not been adequately examined. This study aims to investigate the association between pre-existing diabetes, with and without complications, and CRC mortality.
Methods
Medicare beneficiaries 67 years and older diagnosed with CRC between 2002 and 2011 were studied using the Surveillance, Epidemiology, and End Results (SEER)-Medicare datasets. Pre-existing diabetes was ascertained using validated algorithms. Cox proportional hazards models were used to compare all-cause and CRC-cause-specific death risk differences in relation to prior diabetes diagnosis and diabetes severity (with and without complications) with adjustment for relevant patient demographics and disease characteristics.
Results
Analyses included 93,710 CRC patients. Among the study population, 22,155 (24%) had diabetes prior to CRC diagnosis and 4% had diabetes-related complications (neuropathy, nephropathy, retinopathy, or peripheral circulatory disorders). All-cause CRC mortality was significantly higher among diabetic patients compared with non-diabetic patients (hazard ratio (HR) = 1.20; 95% confidence interval (CI) = 1.17–1.23). The results were more pronounced for diabetes with complications (HR = 1.47; 95% CI = 1.34–1.54). Diabetic patients with complications were 16% more likely to die of colorectal cancer compared with patients without diabetes (HR = 1.16; 95% CI = 1.08–1.25).
Conclusion
Pre-existing diabetes contributes to poorer all-cause mortality among CRC patients and increased mortality from CRC among those with diabetes and complications. Opportunities exist to incorporate diabetes prevention and management interventions during CRC treatment phases among older adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) and type 2 diabetes mellitus (referred to as diabetes) are major causes of morbidity and death in the Unites States (U.S.) [1, 2]. Both diseases are preventable through lifestyle changes, and their complications can be attenuated through screening and early detection [3, 4]. CRC and diabetes share many common risk factors that characterize the Western lifestyle such as poor eating habits and prolonged sedentary behavior [5, 6]. Colorectal cancer is the second leading cause of cancer death among older adult (i.e., age 65 years and older) males and third leading cause of cancer death among older adult females [2]. More than 25% of the older adults suffer from diabetes [7]. Consequently, diabetes is a common comorbid condition among CRC patients, particularly among older adults, with estimated co-occurrences ranging from 5 to 26% [8,9,10,11]. Despite these high rates of comorbidity, the effects of diabetes on CRC outcomes have not been adequately examined, with less being known about the potential influence of diabetes on CRC risk of death and associated risk variations by diabetes severity (complications vs. no complications).
Study purpose
The purposes of this study were to, first, investigate whether diabetes is an independent predictor of poorer survival from all-cause and CRC-specific mortality and second, assess whether variations of CRC survival outcomes exist by diabetes complication status. We hypothesized that mortality risk among CRC patients is higher among those with pre-existing diabetes with and without complications, compared with CRC patients without diabetes.
Methods
Data source and cohort
The Surveillance, Epidemiology, and End Results (SEER)-Medicare linked datasets were used for this study [12]. The SEER cancer registries include 20 U.S. geographic areas and covers approximately 28% of the domestic population [13]. The National Cancer Institute, in partnership with the Centers for Medicare and Medicaid Services (CMS), performs linkage of the SEER data with Medicare claims for Medicare beneficiaries every 2 years, and each linkage successfully matches 95% SEER cases for persons age 65 years and older to their Medicare enrollment and claims files [14]. Both SEER and CMS actively follow patients: SEER registries follow cancer patients and record death/alive status at the end of each data submission while CMS reports information about all beneficiaries from entitlement to death regardless of place of residence.
Demographic and clinical information were extracted for each person from the Patient Entitlement and Diagnosis Summary file (PEDSF) SEER file. Example variables of interest included age, date of death, sex, race, state of residence, date of diagnosis, survival in months, stage at diagnosis, and source of diagnosis. Medicare enrollment information and clinical claims data were extracted from the Medicare Provider Analysis and Review (MEDPAR) for inpatient stays and from the Outpatient Claims file for outpatient stays.
The cohort under study included older adult patients aged 67 years and older diagnosed with CRC between 2002 and 2011. The age was limited to 67 years and older to allow at least 2 years of claims to be analyzed. Patients had to have 24 months continuous enrollment in Part A and Part B to be included [15]. Patients were excluded if the month of CRC diagnosis was missing or if CRC was diagnosed at autopsy or by death certificate. To classify CRC diagnosis, CRC International Classification of Diseases for Oncology 3rd edition (ICD-O-3) codes C18.0–C18.9, C19.9, and C20.9 were used.
Outcomes and predictors
Death risk was assessed using all-cause mortality and CRC-cause-specific mortality. Information about date of death is tracked in both SEER and Medicare enrollment files. Therefore, subjects were included in analyses if their month of death was the same in both files or only differed by a maximum of 1–3 months. Survival in months is provided by the SEER program based on active follow up. Patients were censored if they were “alive” at the cut-off date (December 31, 2011) or if they died after the cut-off date. In addition, for cause-specific mortality, patients were censured if a non-CRC cause of death was documented prior to the cut-off date.
Pre-existing diabetes was ascertained from the Medicare inpatient and outpatient claims using a validated algorithm (algorithm #1) with 74.4% sensitivity and 97.5% specificity [16]. Based on the Hebert algorithm, claims were searched 24 months prior to a CRC diagnosis. The identification period was expanded to 3 months after CRC diagnosis to capture patients who may not have had a healthcare encounter before their cancer diagnosis [15]. To avoid “rule out” diagnoses, a flag was assigned to a record if a diabetes diagnosis code appeared in a single hospital claim and two or more outpatient claims separated by more than 30 days. Applying the rule out algorithm helps to avoid overestimating conditions when they are identified from claims. Medication was not included in the algorithm for identifying patients with diabetes because CMS started covering medication in Part D only since 2006. To classify type 2 diabetes diagnoses, the following ICD-9-CM codes were used: 250.00, 50.01, 250.02, 250.03, 250.10, 250.11, 250.12, 250.13, 250.20, 250.21, 250.22, 250.23, 250.30, 250.31, 250.32, and 250.33. Diabetes with complications included neuropathy, nephropathy, retinopathy, or peripheral circulatory disorders. Patients with no diabetes diagnosis served as the referent group in all analyses.
Demographic covariate variables controlled for in analyses were classified at the time of CRC diagnosis. The covariates included age groups (67–75, 76–85, and 85 years old and older), marital status (single, married, separated/divorced, widowed, and unknown), and race/ethnicity (White non-Hispanic, Black non-Hispanic, Hispanic, non-Hispanic Asian Pacific Islander, and non-Hispanic American Indian/Alaska Native). Poverty level, was computed from the 2000 U.S. Census and the 2012 American Community Survey for all cases using median household income, then ranked and grouped into quartiles (low, medium, medium high, and high poverty level) [17].
The comorbidity score, which excludes diabetes, was calculated based on an updated version of the Charlson index by the National Cancer Institutes, which is a cancer-specific index including 14 conditions (e.g., acute myocardial infarction, congestive heart failure, and cerebrovascular diseases) and excludes solid tumors, leukemias, and lymphomas [18].
Clinical covariates included tumor site (proximal colon consisting of the cecum to the splenic flexure, distal colon consisting of the descending and sigmoid colon, colon not otherwise specified (NOS), and rectum), histology (adenocarcinomas, carcinomas, and carcinomas NOS), stage at diagnosis (localized, regional, and distant), and comorbidity score (no comorbidity, 1 comorbidity, and 2 or more comorbidities). Treatment was not controlled for in analyses because treatment decisions are affected by patient cancer stage and concurrent existing comorbid conditions. We controlled for both these confounding variables.
Statistical analyses
Descriptive analyses were used to compare demographic and clinical information for colorectal cancer patients by their diabetes status and the presence of diabetes-related complications. Chi-square tests assessed differences between groups for categorical variables. Significant differences in survival were tested with the log-rank tests. All-cause and CRC-cause-specific death risk differences were compared using Cox proportional hazards models, and hazard ratios (HR) were compared in relation to prior diabetes diagnosis and diabetes complications status. All models were adjusted for relevant covariates. Tied data were adjusted using the Efron approximation. The proportional hazards assumption was tested and met based on the graphed Schoenfeld residuals for predictors and covariates [19]. The 95% confidence intervals (CI) for HRs were generated and reported. All statistical analyses were performed using version 9.4 of the SAS statistical software (SAS Institute).
Results
The study analyses included 93,710 CRC patients followed for a total of 320,087 person-years. The mean age of the study population was 78 (± 7) years and the median age was 77 years (range 67 to 108 years). Among the study population, 22,155 (24%) had diabetes prior to CRC diagnosis. Among those with diabetes, 17% (3,827) had diabetes-related complications. A significantly larger proportion of diabetic patients were less than 85 years old, female, and White (Table 1). A large proportion of diabetic patients also lived in medium to high poverty level areas, had at least one or more comorbidities, and had tumors in the proximal colon (Table 2).
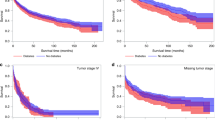
At the end of the study period, 44,688 subjects- representing 48% of the initial cohort- died of all causes. CRC-specific deaths, a sub-part of all deaths, amounted to 26,037 deaths. Among patients who died of all causes, 20% had diabetes without complications and 5% had diabetes with complications. Among those who died specifically of CRC, 18% had diabetes without complications and 4% had diabetes with complication (Table 3). The median survival for all-cause mortality was 61 months (95% CI = 60–62). The overall 5-year survival rate for the study cohort was 51% (Standard error (SE) = 0.00186). Diabetes with complications had the most unfavorable crude survival followed by those with diabetes without complications (Kaplan Meier log-rank test P < 0.0001).
In the univariate Cox regression model, CRC patients with diabetes had 21% increased risk of mortality from all causes (HR = 1.21; 95% CI = 1.18–1.23). The mortality risk was particularly higher among diabetic patients with complications (HR = 1.69; 95% CI = 1.62–1.76) (Table 4, Model 1). Significant results were only observed for diabetes with complications in CRC-cause-specific mortality (HR = 1.14; 95% CI = 1.07–1.22). Similar results were observed when adjusted for age and comorbidities, although the magnitude of effects attenuated after the introduction of comorbidities (Table 4, Models 2 and 3).
In the fully adjusted model, colorectal cancer patients were more likely to die if they had diabetes (HR = 1.20; 95% CI = 1.17–1.23), particularly those with complications (HR = 1.47; 95% CI = 1.34–1.54), compared with those with no prior diabetes diagnosis. Diabetes patients with complications were 16% more likely to die of CRC compared with patients without diabetes (HR = 1.16; 95% CI = 1.08–1.25) (Table 4, Model 4).
Discussion
This study shows that the impact of diabetes on survival rates among older adult CRC patients is substantial. Moreover, not unexpected, the specific groups of CRC patients with diabetes and complications had almost a 50% higher risk of all-cause death and a 16% higher risk of CRC-cause-specific death. These findings are unique in that most studies that examine the association of CRC and diabetes have not parsed out the effects of diabetes status by severity [20,21,22,23,24,25,26,27,28,29].
This study found a significant association between pre-existing diabetes and all-cause mortality among a cohort of older adult CRC patients. In a population-based study, Groß et al. found a 23% increase in risk from all-cause mortality among patients with comorbid diabetes [21]. Patients in a clinical trial had poorer prognosis if they had diabetes at the time of CRC diagnosis; a 42% increased risk of all-cause death [22]. Similarly, patients with pre-existing diabetes from the Connecticut cancer registry were found to have a 38% elevated risk of all-cause mortality [26]. Bella et al. found a 41% increased risk of all-cause mortality among CRC adult Italian patients with comorbid diabetes irrespective of sex or sub-site [25]. Other similar results have been reported in systematic reviews [30, 31].
In the present study, although we found a significant association between pre-existing diabetes and CRC-cause-specific mortality, the effect size was minimal. This small effect aligns with the lack of concordance within the current literature, which indicates mixed results about the association of pre-existing diabetes with CRC-cause-specific mortality. For instance, for non-U.S. populations [2, 25, 32] and in some meta-analyses [27, 33], authors have reported significant relationships between comorbid diabetes and CRC mortality. In contrast, other researchers did not identify elevated risk for CRC mortality among diabetic patients [26, 31].
The exact pathophysiological mechanism through which diabetes may affect CRC prognosis is not clear; however, some proposed pathways include hyperinsulinemia and hyperglycemia. These are known factors that contribute to increased risk of cancer and tumor metastasis [29, 34, 35], and may also directly affect the outcomes of CRC. Moreover, diabetes may increase the risk of CRC recurrence, which may partly contribute to the observed poorer prognosis among diabetic patients with CRC [22, 28, 33].
In addition, diabetes might increase general mortality as well as death from diabetes-related diseases (e.g., stroke, ischemic heart disease, hypertension, chronic renal failure). A study of multimorbidity and survival among persons with CRC found that among CRC deaths, 9% were attributable to congestive heart failure and more than 5% were attributable to chronic obstructive pulmonary disease [21]. Another study using a large U.S. cohort found a twofold increase of death from cardiovascular diseases among patients with self-reported diabetes and CRC [27]. Moreover, there are potential indirect influences of diabetes on cancer management and treatment. Comorbid diabetes among CRC patients might influence treatment decisions, treatment response, and treatment-related side effects. Researchers found that older patients with diabetes and other comorbidities were less likely to see an oncologist in the first six months after diagnosis [36, 37], less likely to start and/or complete recommended CRC adjuvant chemotherapy [38, 39] or neoadjuvant therapy for metastasized cancer [39], and less likely to receive aggressive cancer treatment [20]. These treatment disparities may be attributed to increased cancer treatment-related side effects, real or perceived by clinicians, which indicates a potential clinician bias towards cancer patients with multimorbidity [22].
As seen in the current study, patients with diabetes-related complications were more likely to die of CRC. Reasons for this effect have not been fully explored. However, this finding suggests that poor diabetes control is unfavorable for CRC patients, and patients could benefit from controlling diabetes and preventing its complications. Uncontrolled diabetes is a known independent risk factor for diabetes complications [40]; hence, clinicians play a large role in aiding their patients to manage their diabetes through regular HbA1c testing, education about lifestyle changes, and medication. For instance, it has been demonstrated that cancer patients who receive diabetes education are less likely to visit emergency departments, have fewer hospital admissions, and are more likely to manage their diabetes with more frequent HbA1c tests [41].
To complement clinical care provided by physicians, CRC patients with diabetes may also benefit from evidence-based chronic disease self-management education programs delivered in community settings. Such programs are widely available nationwide [42, 43] and are effective to reduce physical and mental health ramifications [44, 45] as well as direct costs associated with emergency room visits and hospitalizations [46].
Strengths and limitations
The strength of this study lies in its inclusion of a large number of patients from a nationally representative database. The SEER-Medicare data offer a combination of clinical information from the cancer registries as well as diagnoses and procedures from the Medicare claims data. These data are population-based and enable the tracking of patients longitudinally. The integration of various data elements and ability to follow patients throughout the duration of their cancer experience is important in that it facilitates in-depth studies such as these to examine aspects associated with survivorship and mortality.
Findings from this study should be interpreted considering certain limitations. Administrative data are not inherently designed for research. Therefore, some information on lifestyle behaviors and patient characteristics were not available for the current study which may have affected the magnitude of the effects. However, other studies that controlled for these factors did not observe a weakening in the association under study [10, 27]. Moreover, colon and rectal cancers behave differently and have different prognosis based on cancer stage. While findings could have been stratified by cancer site, previous research reports no indication of differences in mortality outcomes by diabetes status (i.e., diabetes vs. no diabetes) [47].
Further, identifying diabetes from administrative data introduced additional limitations. First, diabetes cases may have been overlooked if the individual did not have a health system encounter. To mitigate this limitation, we allowed a 24-month period before CRC diagnosis and 3 months after CRC diagnosis to identify previously undiagnosed diabetes [15]. Second, the dataset lacked information about cases with prediabetes (impaired fasting glucose and/or impaired glucose tolerance); therefore, hazard ratios in the current study might have been underestimated. This remains a concern because the prevalence of prediabetes is on the rise in the U.S. [1]. Third, we were unable to identify diabetes duration; however, this has not shown to be impactful of survival in similar studies [27].
Conclusion
In summary, this study used population-based data and the findings indicate that pre-existing diabetes contributes to poorer all-cause survival among patients with CRC and increased mortality from CRC for patients with diabetes and associated complications. These findings are relevant in the context of the rising prevalence of diabetes among the aging U.S. population. Because CRC and diabetes (and their comorbidity) are more prevalent among older adults, these complex patients may require additional clinical interactions and support, which will entail the development of care plans that are interdisciplinary and take into consideration the added burden of diabetes among CRC patients. In addition, findings also underscore lack of understanding about how diabetes affects CRC outcomes. Particular attention is needed for patients with diabetes complications as they suffer from the worst outcomes. Increased focus on diabetes education, diabetes self-management, and improved diabetes control are critical to improve survival among colorectal patients with comorbid diabetes.
References
Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metrics 8(1):29. https://doi.org/10.1186/1478-7954-8-29
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21395
Calonge N, Petitti DB, DeWitt TG, Dietrich AJ, Gregory KD, Harris R et al (2008) Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 149(9):627–637. https://doi.org/10.7326/0003-4819-149-9-200811040-00243
Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Aunola S, Cepaitis Z, Moltchanov V, Hakumäki M, Mannelin M, Martikkala V, Sundvall J, Uusitupa M (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344(18):1343–1350. https://doi.org/10.1056/NEJM200105033441801
Harriss DJ, Atkinson G, Batterham A, George K, Tim Cable N, Reilly T, Haboubi N, Renehan AG, The Colorectal Cancer, Lifestyle, Exercise And Research Group (2009) Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Color Dis 11(7):689–701. https://doi.org/10.1111/j.1463-1318.2009.01767.x
Reis JP, Loria CM, Sorlie PD, Park Y, Hollenbeck A, Schatzkin A (2011) Lifestyle factors and risk for new-onset diabetes: a population-based cohort study. Ann Intern Med 155(5):292–299. https://doi.org/10.7326/0003-4819-155-5-201109060-00006
Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS (2012) Diabetes in older adults. Diabetes Care 35(12):2650–2664. https://doi.org/10.2337/dc12-1801
Campbell PT, Deka A, Jacobs EJ, Newton CC, Hildebrand JS, McCullough ML et al (2010) Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 139(4):1138–1146. https://doi.org/10.1053/j.gastro.2010.06.072
Deng L, Gui Z, Zhao L, Wang J, Shen L (2012) Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig Dis Sci 57(6):1576–1585. https://doi.org/10.1007/s10620-012-2055-1
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159(12):1160–1167. https://doi.org/10.1093/aje/kwh161
Chiao EY, Nambi PV, Naik AD (2010) The impact of diabetes process and outcome quality measures on overall survival in patients with co-morbid colorectal cancer. J Cancer Surviv 4(4):381–387. https://doi.org/10.1007/s11764-010-0141-y
Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF (2002) Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40(8):18 https://www.jstor.org/stable/3767919. Accessed 1 Jan 2019
Research Data (1973–2010) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission. [Internet].; 2013 [cited July 2013]. Available from: www.seer.cancer.gov
SEER-Medicare: how the SEER and Medicare data are linked [Internet].; 2018 [cited January 1, 2019]. Available from: https://healthcaredelivery.cancer.gov/seermedicare/overview/linked.html. Accessed 1 Jan 2019
Yang Y, Mauldin PD, Ebeling M, Hulsey TC, Liu B, Thomas MB, Camp ER, Esnaola NF (2013) Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer 119(8):1512–1520. https://doi.org/10.1002/cncr.27923
Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM (1999) Identifying persons with diabetes using Medicare claims data. Am J Med Qual 14(6):270–277. https://doi.org/10.1177/106286069901400607
Coker AL, Du XL, Fang S, Eggleston KS (2006) Socioeconomic status and cervical cancer survival among older women: findings from the SEER–Medicare linked data cohorts. Gynecol Oncol 102(2):278–284. https://doi.org/10.1016/j.ygyno.2005.12.016
Klabunde CN, Legler JM, Warren JL, Baldwin L, Schrag D (2007) A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 17(8):584–590. https://doi.org/10.1016/j.annepidem.2007.03.011
Hess KR (1995) Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 14(15):1707–1723
van de Poll-Franse, Lonneke V, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JWW, Haak HR (2007) Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer 120(9):1986–1992. https://doi.org/10.1002/ijc.22532
Gross CP, Guo Z, McAvay GJ, Allore HG, Young M, Tinetti ME (2006) Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc 54(12):1898–1904. https://doi.org/10.1111/j.1532-5415.2006.00973.x
Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB III, Fuchs CS (2003) Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 21(3):433–440. https://doi.org/10.1200/JCO.2003.07.125
Jullumstr E, Kollind M, Lydersen S, Edna T (2009) Diabetes mellitus and outcomes of colorectal cancer. Acta Oncol 48(3):361–367. https://doi.org/10.1080/02841860802637765
Huang Y, Lin J, Chen W, Lin T, Yang S, Jiang J et al (2011) Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. J Cancer Res Clin Oncol 137(2):211–220. https://doi.org/10.1007/s00432-010-0879-7
Bella F, Minicozzi P, Giacomin A, Crocetti E, Federico M, De Leon MP et al (2013) Impact of diabetes on overall and cancer-specific mortality in colorectal cancer patients. J Cancer Res Clin Oncol 139(8):1303–1310. https://doi.org/10.1007/s00432-013-1439-8
Polednak AP (2006) Comorbid diabetes mellitus and risk of death after diagnosis of colorectal cancer: a population-based study. Cancer Detect Prev 30(5):466–472. https://doi.org/10.1016/j.cdp.2006.07.003
Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT (2011) Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 30(1):53–59. https://doi.org/10.1200/JCO.2011.38.0303
Feng J, Zhou X, Mao W (2011) Prognostic analysis of colorectal cancer patients with diabetes mellitus in China–the experience of a single institution. Adv Clin Exp Med 20:473–480
Bao Y, Nimptsch K, Meyerhardt JA, Chan AT, Ng K, Michaud DS, Brand-Miller JC, Willett WC, Giovannucci E, Fuchs CS (2010) Dietary insulin load, dietary insulin index, and colorectal cancer. Cancer Epidemiol Biomarkers Prev 19(12):3020–3026. https://doi.org/10.1158/1055-9965.EPI-10-0833
Barone BB, Yeh H, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 300(23):2754–2764. https://doi.org/10.1001/jama.2008.824
Stein KB, Snyder CF, Barone BB, Yeh H, Peairs KS, Derr RL et al (2010) Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci 55(7):1839–1851. https://doi.org/10.1007/s10620-009-0944-8
Huang Y, Lin J, Chen W, Lin T, Yang S, Jiang J et al (2011) Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. J Cancer Res Clin Oncol 137(2):211–220. https://doi.org/10.1007/s10620-009-0944-8
Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G (2013) Diabetes and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum 56(11):1304–1319. https://doi.org/10.1097/DCR.0b013e3182a479f9
Tsai C, Giovannucci EL (2012) Hyperinsulinemia, insulin resistance, vitamin d, and colorectal cancer among whites and African Americans. Dig Dis Sci 57(10):2497–2503. https://doi.org/10.1007/s10620-012-2198-0
Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86(3):842S–842S. https://doi.org/10.1093/ajcn/86.3.836S
Luo R, Giordano SH, Freeman JL, Zhang D, Goodwin JS (2006) Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist 11(9):1025–1033. https://doi.org/10.1634/theoncologist.11-9-102539
Gross CP, McAvay GJ, Guo Z, Tinetti ME (2007) The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer 109(12):2410–2419 10.1002/cncr.22726
Bradley CJ, Given CW, Dahman B, Fitzgerald TL (2008) Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med 168(5):521–529. https://doi.org/10.1001/archinternmed.2007.82
O’Grady MA, Slater E, Sigurdson ER, Meropol NJ, Weinstein A, Lusch CJ, Sein E, Keeley P, Miller B, Engstrom PF, Cohen SJ (2011) Assessing compliance with national comprehensive cancer network guidelines for elderly patients with stage III colon cancer: the Fox Chase Cancer Center Partners’ initiative. Clin Colorectal Cancer 10(2):113–116. https://doi.org/10.1016/j.clcc.2011.03.007
Stratton IM, Cull CA, Adler AI, Matthews DR, Neil H, Holman RR (2006) Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 49(8):1761–1769. https://doi.org/10.1007/s00125-006-0297-1
Irizarry L, Li QE, Duncan I, Thurston AL, Fitzner KA, Edwards BJ, McKoy-Bent JM, Tulas KM, McKoy JM (2013) Effects of cancer comorbidity on disease management: making the case for diabetes education (a report from the SOAR program). Popul Health Manag 16(1):53–57. https://doi.org/10.1089/pop.2012.0028
Smith M, Towne S, Herrera-Venson A, Cameron K, Horel S, Ory M, Gilchrist C, Schneider E, DiCocco C, Skowronski S (2018) Delivery of fall prevention interventions for at-risk older adults in rural areas: findings from a national dissemination. Int J Environ Res Public Health 15(12):2798. https://doi.org/10.3390/ijerph15122798
Ory MG, Smith ML, Patton K, Lorig K, Zenker W, Whitelaw N (2013) Self-management at the tipping point: reaching 100,000 Americans with evidence-based programs. J Am Geriatr Soc 61(5):821–823. https://doi.org/10.1111/jgs.12239
Ory MG, Ahn S, Jiang L, Smith ML, Ritter PL, Whitelaw N, Lorig K (2013) Successes of a national study of the chronic disease self-management program: meeting the triple aim of health care reform. Med Care 51(11):992–998
Ory MG, Ahn S, Jiang L, Lorig K, Ritter P, Laurent DD, Whitelaw N, Smith ML (2013) National study of chronic disease self-management: six-month outcome findings. J Aging Health 25(7):1258–1274. https://doi.org/10.1177/0898264313502531
Ahn S, Basu R, Smith ML, Jiang L, Lorig K, Whitelaw N, Ory MG (2013) The impact of chronic disease self-management programs: healthcare savings through a community-based intervention. BMC Public Health 13(1):1141. https://doi.org/10.1186/1471-2458-13-1141
Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G (2013) Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum 56(11):1304–1319. https://doi.org/10.1097/DCR.0b013e3182a479f9
Acknowledgments
We thank Sani H. Kizilbash, MBBS, MPH for his counseling on data methods and statistical programs.
Funding
This work was partially supported by Exito!- the National Cancer Institute (grant no. 1R25CA134301-01A2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights and informed consent
The study did not involve research on human subjects and it did not require informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The funding source had no involvement in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El brahimi, S., Smith, M.L. & Pinheiro, P.S. Role of pre-existing type 2 diabetes in colorectal cancer survival among older Americans: a SEER-Medicare population-based study 2002–2011. Int J Colorectal Dis 34, 1467–1475 (2019). https://doi.org/10.1007/s00384-019-03345-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03345-8