Abstract
Purpose
Ventral mesh rectopexy (VMR) is an established, minimally invasive, nerve-sparing procedure for the treatment of various symptomatic morphological changes in the posterior pelvic compartment. We present the short-term functional outcome and patient satisfaction after laparoscopic and robotic VMR with biological mesh.
Methods
We analyzed data from 123 patients who underwent laparoscopic ventral mesh rectopexy (LVMR) or robotic ventral mesh rectopexy (RVMR) from August 2012 to January 2017. Included in these data were patient demographics, intra- and postoperative findings, Cleveland Clinic Constipation Score (CCCS), Obstructed Defecation Score Longo (ODS), Cleveland Clinic Incontinence Score (CCIS), and patient satisfaction as measured by visual analog scale (0–10).
Results
Improvements in CCCS, CCIS, and ODS were statistically significant at 6 and 12 months (p < 0.001). Patient satisfaction was excellent at 6 and 12 months (8.2/10 and 8.3/10, respectively). The overall complication rate was 14%, with a major complication rate of 2%. No mesh-related complications were observed. The need for surgical re-intervention because of relapse, symptom persistence or recurrence, or new symptoms was 3%. Outcome appears to be similar between LVMR and RVMR.
Conclusions
Both LVMR and RVMR with biological mesh are safe and effective in reducing symptoms, as measured by CCCS, CCIS, and ODS, and patient satisfaction is high.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In addition to its original use in full-thickness external rectal prolapse, laparoscopic ventral mesh rectopexy (LVMR) is now an established procedure for the treatment of morphological changes in the posterior pelvic compartment associated with functional impairment (e.g., rectocele, intussusception, enterocele, sigmoidocele, and pelvic-floor insufficiency) [1,2,3]. Its aims are to correct anatomy and thus improve symptoms of obstruction and fecal incontinence [2].
An essential advantage of LVMR is the lack of necessity for posterior mobilization of the rectum. The LVMR approach preserves the autonomic nerves and thus prevents significant new-onset constipation owing to rectal denervation [4, 5]. Moreover, it offers all the known advantages of minimally invasive surgery, such as reduced morbidity and shorter hospitalization [6], while demonstrating a recurrence rate equivalent to open surgery’s [7]. In selected patients, even same-day discharge is reported to be feasible and safe [8].
As yet, however, some questions remain. With synthetic mesh, complications such as infection, mesh erosion, fistula formation, and dyspareunia have been described [9,10,11]. Hypothetically, biological mesh may be associated with fewer complications [9], and evidence suggests that it is efficient and safe [12] for both LVMR and robotic ventral mesh rectopexy (RVMR) [13,14,15].
Data on functional outcome after ventral rectopexy with biological mesh are scarce, as are those on patient satisfaction: one study evaluated patients’ symptoms after LVMR by interview [16] and another measured quality of life after LVMR and RVMR with SF-36 [13].
The present study aims to evaluate both functional results and patient satisfaction after LVMR and RVMR with biological mesh.
Methods
Laparoscopic or robotic ventral rectopexy with a biological mesh was performed in 123 patients from August 2012 to January 2017. Data were prospectively obtained and retrospectively analyzed.
For preoperative diagnostic investigations, all patients underwent a clinical examination that included rigid proctoscopy and, except for those with full-thickness rectal prolapse (14%), all underwent MRI-defecography (or conventional defecography if MRI was contraindicated). Anorectal manometry, colonoscopy, and colonic transit time were performed selectively when indicated.
All operative procedures were performed by the same team of two surgeons. For the robotic approach, the da Vinci Si HD system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was used. For the first cases of robotic surgery, patients were limited to those with no previous surgery and a BMI below 30 kg/m2. Subsequently, there were no specific selection criteria. For the biological mesh, an 18- × 4-cm strip of 1-mm-thick Permacol™ was used in 103 and a 20- × 4-cm Biodesign® mesh in 20. Postoperatively, all patients were instructed to avoid extensive straining at stool and to use laxatives for 3 months.
All rectopexies performed were included in the analysis. The patients represent a consecutive series. Data were collected on patient demographics, previous surgery, preoperative diagnostic studies, intra- and postoperative findings, complications, and hospitalization time. Functional outcome was assessed by Cleveland Clinic Constipation Score (CCCS), Obstructed Defecation Score Longo (ODS), and Cleveland Clinic Incontinence Score (CCIS) preoperatively and at 6 and 12 months. The questionnaire was handed to the patients; appropriateness in filling in answers and completeness were checked by staff not involved in the study. Patients were also clinically examined at 6 and 12 months. Patient satisfaction was measured by a visual analog scale from 0 (none) to 10 (full satisfaction) at 6 and 12 months’ follow-up.
Surgical technique
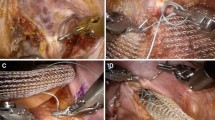
The technique used was that described by D’Hoore, Cadoni and Penninckx [1] with few modifications. For RVR, three 8-mm ports and one 12-mm assistant port were placed with an adequate distance between to prevent robotic arm interference and for LVR two to three 5-mm and one 12-mm port. Dissection was routinely performed with a right lateral perirectal peritoneal incision and hockey-stick distal shape to the left and dissection down to the pelvic floor with only ventral exposure of the rectal wall. Distally, the mesh was fixed at the ventral rectal wall with three suture lines (3-3-2 stitches) of 2/0 Ethibond. The proximal end of the mesh was fixed to the sacral promontory with the ProTack fixation device (Covidien, Mansfield, MA, USA). The right lateral peritoneal incision was closed with a continuous suture running from distal to proximal to cover the mesh and recreate the pouch of Douglas. The operative technique was similar for RVMR and LVMR.
Before starting the procedure, the patient was placed in the Trendelenburg position. In female patients without hysterectomy, the uterus was retracted upward to the anterior abdominal wall with a stitch that went through the abdominal wall and the ligamentum latum uteri on the one side and back on the other side.
Statistical analysis
Data analysis was performed with SPSS software. Comparisons of metric and ordinal data were calculated with the Student’s t test or Mann-Whitney U test. The chi-square test was used for categorical data. Statistical significance was set at p < 0.05.
Results
Demographics
Of the 123 patients (median age 63.0 years) who underwent VMR, RVMR was performed in 23 and LVMR in 100. The majority of patients were women (95%), and the average BMI was 26.2 kg/m2. The mean number of previous abdominal surgeries was 1.7, with hysterectomy being the most frequent (N = 69, or 59% of the female patients) (Table 1).
There were no differences in gender, BMI, or previous abdominal surgery between the groups, but age was significantly lower in those who had RVMR.
Indications for surgery
The leading symptom (see Table 2) was obstructed defecation (OD) in 43% of patients and fecal incontinence (FI) in 23%; symptoms of both were reported in 26%. Other symptoms such as rectal bleeding predominated in 8%.
In most patients (67%), a combination of various morphological alterations of the posterior compartment of the pelvic organs was found: descending pelvic floor (81%), rectocele (66%), enterocele (27%), intussusception (14%), and full-thickness external rectal prolapse (14%). In 20% (n 24), patients had recurrent symptoms after previous interventions (with three patients after two previous operations): rectopexy in 8, rectopexy with resection in 5, STARR procedure in 10, Altemeier in 3, and Rehn-Delorme in 1.
Surgery
Permacol™ biological mesh was used in 84% (LVMR 80/100; RVMR 23/23) and Biodesign® in 16% (LVMR 20/100). Length of postoperative stay was 7.3 days. The conversion rate in the LVMR group was 0, and in the RVMR group 4%, as one patient required conversion to the laparoscopic approach because of massive adhesions in the pelvis after a previous operation.
Complications
Complications occurred in 17 patients (14%) (LVMR 15/100; RVMR 2/23) and did not differ significantly in grade between groups (Table 3). Most complications (88%) were grades I and II of the Clavien-Dindo classification. Minor complications included urinary tract infections (four patients), hypertension (three patients), abdominal wall hematoma (two patients), fever of undetermined origin (two patients), abdominal wall rash (one patient), nausea (one patient), lower leg edema (one patient), and pleural effusion (one patient). Two patients had to undergo re-operation: one developed acute appendicitis and one a small-bowel fistula. There were no cases of mesh infection or erosion, and there was no mortality.
Re-interventions for recurrence or persistence and relapse of symptoms
Through the entire follow-up of 28.8 months (mean, range 6–58), four patients (3%) underwent additional interventions for morphological or functional reasons related to their initial presentation: One patient, who had presented with full-thickness rectal prolapse, developed a recurrence 12 months after LVMR consequent to a forceful colonoscopy and underwent re-LVRM. This patient developed a second recurrence and underwent a third VMR, now with a synthetic mesh. One male patient, after removal of full-thickness rectal prolapse, requested a stoma because of persistent local inflammation of the rectum combined with evacuation disorders. One female patient reported persistent obstructed defecation after LVRM and underwent laparoscopic exploration. A sigmoidocele was found, which was successfully corrected by sigmoidopexy. Another patient underwent successful sacral nerve stimulation for persistent fecal incontinence. One patient required colpopexy for de-novo vaginal vault prolapse. Two further patients with symptoms of relapse were treated conservatively.
Functional outcome
Symptom improvement was high, as evidenced by a highly significant improvement in CCCS, ODS, and CCIS at 6 and 12 months postoperatively (p < 0.001) (Fig. 1).
The gradients of CCCS, CCIS, and ODS with stratification to predominant symptom, morphological disorder, and surgical approach are shown in Tables 4, 5, and 6. Patients with obstructed defecation showed a significant improvement of CCCS and ODS at 6 and 12 months (p < 0.001). CCIS results in patients with anal incontinence were also significantly improved at 6 and 12 months (p < 0.001 and p = 0.025, respectively). Patients with combined obstructed defecation and anal incontinence had a significant improvement of CCIS and ODS at 6 and 12 months (p = 0.004, p = 0.028, and p < 0.001, p = 0.018, respectively). Stratified to morphological changes, CCCS, CCIS, and ODS were significantly improved in patients with descending perineum and rectocele at 12 months and CCCS and CCIS were significantly improved in patients with enterocele at 12 months, but not in patients with intussusception and full-thickness rectal prolapse. Surgical technique did not affect outcome scores.
In the patients in whom fecal incontinence was the leading symptom—all of whom showed an improvement in CCIS—deterioration of fecal incontinence symptoms did not occur nor did de-novo obstructed defecation arise. In patients with obstructed defecation as the leading symptom, 86.7% had an improvement in CCCS and 80.0% in ODS at 12 months’ follow-up; 13.3% (CCCS) and 10.0% (ODS) reported a worsening. There was no de-novo onset of FI in this group.
In the group in whom combined obstructed defecation and fecal incontinence predominated, 95.2% reported an improvement in CCIS, 72.7% in CCCS, and 81.8% in ODS. A worsening of symptoms was found in none on CCIS, in 22.7% on CCCS, and in 13.6% on ODS. A de novo onset of either symptom did not occur.
Patient satisfaction
Patient satisfaction (see Table 7) was 8.2 (of 10) at 6 months and 8.3 at 12 months and revealed no difference between techniques.
Outcome of coexisting urinary symptoms
In 47 patients who presented preoperatively with micturition disorders—urinary incontinence (66%) and urinary emptying (28%) being the most frequent—53% reported improvement after VMR, of whom two-thirds described complete relief. One patient reported postoperative worsening of urinary incontinence. No new-onset micturition disorders arose (Table 8).
Discussion
Minimally invasive VMR is now commonly used for many forms of posterior pelvic organ prolapse. However, the evidence for efficacy and choice of mesh remains limited [12]. This report is the largest series of VMR with biological mesh and only the second on its use in RVMR. Our results confirm and add to evidence that VMR with biological mesh is safe and results in significant symptom improvement and high patient satisfaction in both LVMR and RVMR.
In this series, the indications for VMR were functional disorders associated with various morphological changes of posterior pelvic compartment organs. The strategy for surgical treatment was organ preservation with stabilization of the middle compartment. The choice of surgical treatment for rectocele as one of the more predominant findings remains controversial; however, there is increasing evidence that ventral mesh rectopexy is a valid option [17].
We observed no correlation between a specific morphological condition and a better functional outcome. Moreover, in most cases, there were combinations of pathological morphological findings on imaging.
Published recurrence rates after VMR with synthetic or biological mesh range from 4 to 5% [18], but different lengths of follow-up make comparison challenging. We experienced a similar recurrence rate as reported in other studies, but with a longer mean follow-up (28.8 months). Long-term results are indisputably needed, but this duration may suggest the constancy of biological mesh’s effectiveness over time.
Our complication rate is similar to those published (Table 9) [8, 13, 16, 18,19,20]. Of the 14%, the majority were minor (grades I and II of the Clavien-Dindo classification), with a major complication rate of only 2%.
We chose biological mesh for all our patients because of its lower risk [8, 12, 16, 18, 19, 21, 22], and a recent review found a mesh erosion rate of 0.22%. The majority of mesh complications are reported to arise within the first 12 months [23]. In our series, there were no mesh-related complications.
All scoring systems (CCCS, CCIS, ODS) used to assess symptom severity were significantly reduced at 6 and 12 months postoperatively. We performed a stratification to predominant symptoms, morphological disorders, and surgical approach and found improvement in all three scores. The lack of significance in the presence of an intussusception or rectal full-thickness prolapse probably owes to the low number of cases.
Published data are difficult to compare because of an inconsistency in use of scores and outcome reporting. In our series, postoperative patient satisfaction with functional outcome was assessed by visual analog scale (VAS) and described by patients as high-excellent, reflecting a high quality of life. The VAS, even though not validated, can be considered a semiquantitative measure of individual grading of the various contributing components. Mehmood et al. have confirmed this improvement by their use of the validated SF-36 quality-of-life questionnaire [13].
The encouraging improvement in urinary function with no new onset of symptoms in over half of the patients with micturition disorders suggests that VMR may contribute to stabilization of the entire pelvic floor and multiple pelvic organ systems.
As others have found [13, 15, 24], the outcome of RVMR appears to be similar to that of LVMR. The availability and cost of robotic surgery are currently restrictive factors in its use.
We have demonstrated that VMR (both laparoscopic and robotic) with biological mesh is safe and effective and is associated with significant symptom improvement and high patient satisfaction. This study has some limitations: long-term follow-up is required to confirm these data. Not all patients were available for follow-up (at 6 and12 months the drop-out rate was 29 and 40%, respectively)—an understandable problem in this demographic, but one that could, nevertheless, be relevant to the introduction of a bias. Also, in complex conditions, evaluation with CCCS, CCIS, and ODS may not cast sufficient focus on relevant and specific symptoms.
Conclusion
In the short term, VMR with biological mesh is safe and clinically effective: it is associated with a low complication rate, significant symptom improvement, and high patient satisfaction as evidenced by CCCS, CCIS and ODS. Results did not differ between LVMR and RVMR. Long-term data are needed to confirm these findings.
References
D'Hoore A, Cadoni R, Penninckx F (2004) Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br J Surg 91(11):1500–1505. https://doi.org/10.1002/bjs.4779
Collinson R, Wijffels N, Cunningham C, Lindsey I (2010) Laparoscopic ventral rectopexy for internal rectal prolapse: short-term functional results. Color Dis 12(2):97–104. https://doi.org/10.1111/j.1463-1318.2009.02049.x
Wong M, Meurette G, Abet E, Podevin J, Lehur PA (2011) Safety and efficacy of laparoscopic ventral mesh rectopexy for complex rectocele. Color Dis 13(9):1019–1023. https://doi.org/10.1111/j.1463-1318.2010.02349.x
Samaranayake CB, Luo C, Plank AW, Merrie AE, Plank LD, Bissett IP (2010) Systematic review on ventral rectopexy for rectal prolapse and intussusception. Color Dis 12(6):504–512. https://doi.org/10.1111/j.1463-1318.2009.01934.x
Boons P, Collinson R, Cunningham C, Lindsey I (2010) Laparoscopic ventral rectopexy for external rectal prolapse improves constipation and avoids de novo constipation. Color Dis 12(6):526–532. https://doi.org/10.1111/j.1463-1318.2009.01859.x
Solomon MJ, Young CJ, Eyers AA, Roberts RA (2002) Randomized clinical trial of laparoscopic versus open abdominal rectopexy for rectal prolapse. Br J Surg 89(1):35–39. https://doi.org/10.1046/j.0007-1323.2001.01957.x
Purkayastha S, Tekkis P, Athanasiou T, Aziz O, Paraskevas P, Ziprin P, Darzi A (2005) A comparison of open vs. laparoscopic abdominal rectopexy for full-thickness rectal prolapse: a meta-analysis. Dis Colon Rectum 48(10):1930–1940. https://doi.org/10.1007/s10350-005-0077-x
Powar MP, Ogilvie JW Jr, Stevenson AR (2013) Day-case laparoscopic ventral rectopexy: an achievable reality. Color Dis 15(6):700–706. https://doi.org/10.1111/codi.12110
Smart NJ, Pathak S, Boorman P, Daniels IR (2013) Synthetic or biological mesh use in laparoscopic ventral mesh rectopexy—a systematic review. Color Dis 15(6):650–654. https://doi.org/10.1111/codi.12219
Madiba TE, Baig MK, Wexner SD (2005) Surgical management of rectal prolapse. Arch Surg 140(1):63–73. https://doi.org/10.1001/archsurg.140.1.63
Hurtado EA, Bailey HR, Reeves KO (2007) Rectal erosion of synthetic mesh used in posterior colporrhaphy requiring surgical removal. Int Urogynecol J Pelvic Floor Dysfunct 18(12):1499–1501. https://doi.org/10.1007/s00192-007-0403-z
Alam NN, Narang SK, Köckerling F, Daniels IR, Smart NJ (2015) Rectopexy for rectal prolapse. Front Surg 2:54
Mehmood RK, Parker J, Bhuvimanian L, Qasem E, Mohammed AA, Zeeshan M, Grugel K, Carter P, Ahmed S (2014) Short-term outcome of laparoscopic versus robotic ventral mesh rectopexy for full-thickness rectal prolapse. Is robotic superior? Int J Color Dis 29(9):1113–1118. https://doi.org/10.1007/s00384-014-1937-4
Germain A, Perrenot C, Scherrer ML, Ayav C, Brunaud L, Ayav A, Bresler L (2014) Long-term outcome of robotic-assisted laparoscopic rectopexy for full-thickness rectal prolapse in elderly patients. Color Dis 16(3):198–202. https://doi.org/10.1111/codi.12513
Ramage L, Georgiou P, Tekkis P, Tan E (2015) Is robotic ventral mesh rectopexy better than laparoscopy in the treatment of rectal prolapse and obstructed defecation? A meta-analysis. Tech Coloproctol 19(7):381–389. https://doi.org/10.1007/s10151-015-1320-7
Wahed S, Ahmad M, Mohiuddin K, Katory M, Mercer-Jones M (2012) Short-term results for laparoscopic ventral rectopexy using biological mesh for pelvic organ prolapse. Color Dis 14(10):1242–1247. https://doi.org/10.1111/j.1463-1318.2011.02921.x
Mustain WC (2017) Functional disorders: rectocele. Clin Colon Rectal Surg 30(1):63–75. https://doi.org/10.1055/s-0036-1593425
Sileri P, Franceschilli L, de Luca E, Lazzaro S, Angelucci GP, Fiaschetti V, Pasecenic C, Gaspari AL (2012) Laparoscopic ventral rectopexy for internal rectal prolapse using biological mesh: postoperative and short-term functional results. J Gastrointest Surg 16(3):622–628. https://doi.org/10.1007/s11605-011-1793-2
Sileri P, Capuano I, Franceschilli L, Giorgi F, Gaspari AL (2014) Modified laparoscopic ventral mesh rectopexy. Tech Coloproctol 18(6):591–594. https://doi.org/10.1007/s10151-013-1094-8
Albayati S, Morgan MJ, Turner CE (2017) Laparoscopic ventral rectopexy for rectal prolapse and rectal intussusception using a biological mesh. Color Dis 19(9):857–862. https://doi.org/10.1111/codi.13671
Evans C, Stevenson AR, Sileri P, Mercer-Jones MA, Dixon AR, Cunningham C, Jones OM, Lindsey I (2015) A multicenter collaboration to assess the safety of laparoscopic ventral rectopexy. Dis Colon Rectum 58(8):799–807. https://doi.org/10.1097/DCR.0000000000000402
Balla A, Quaresima S, Smolarek S, Shalaby M, Missori G, Sileri P (2017) Synthetic versus biological mesh-related erosion after laparoscopic ventral mesh rectopexy: a systematic review. Ann Coloproctol 33(2):46–51. https://doi.org/10.3393/ac.2017.33.2.46
Abed H, Rahn DD, Lowenstein L, Balk EM, Clemons JL, Rogers RG, Systematic Review Group of the Society of Gynecologic Surgeons (2011) Incidence and management of graft erosion, wound granulation, and dyspareunia following vaginal prolapse repair with graft materials: a systematic review. Int Urogynecol J 22(7):789–798. https://doi.org/10.1007/s00192-011-1384-5
van Iersel JJ, Paulides TJ, Verheijen PM, Lumley JW, Broeders IA, Consten EC (2016) Current status of laparoscopic and robotic ventral mesh rectopexy for external and internal rectal prolapse. World J Gastroenterol 22(21):4977–4987. https://doi.org/10.3748/wjg.v22.i21.4977
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.E. Matzel is medical adviser to Medtronic.
M. Brunner has received an honorarium for lecturing at a Medtronic workshop.
Statement of human rights
For this type of study, formal consent is not required.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Informed consent
In this study, there is no identifying information of patients.
Rights and permissions
About this article
Cite this article
Brunner, M., Roth, H., Günther, K. et al. Ventral rectopexy with biological mesh: short-term functional results. Int J Colorectal Dis 33, 449–457 (2018). https://doi.org/10.1007/s00384-018-2972-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-018-2972-3