Abstract
Purpose
We evaluate whether the change of carcinoembryonic antigen (CEA) level before and after preoperative chemoradiotherapy (CRT) in rectal cancer affects tumor response and recurrence or not.
Methods
We retrospectively analyzed 1447 rectal cancer patients who underwent preoperative CRT followed by curative surgery. All patients received preoperative radiotherapy of 50.4 Gy in 28 fractions with 5-fluorouracil or capecitabine. Total mesorectal excision was performed 4 to 8 weeks after preoperative CRT. CEA levels were checked before and after CRT. Clinical and pathologic factors were analyzed for tumor response and recurrence.
Results
Post-CRT CEA level (cutoff value, 2.5 ng/mL) was not a significant factor for tumor response on the multivariate analysis (p = 0.095). Patients were categorized according to the pre- and post-CRT CEA level (group A: pre-CRT CEA ≤5 ng/mL; group B: pre-CRT CEA >5 ng/mL and post-CRT CEA ≤2.5 ng/mL; group C: pre-CRT CEA >5 ng/mL and post-CRT CEA >2.5 ng/mL). The relapse-free survival (RFS) at 5 years was significantly higher in group A than in groups B and C (82.6 vs. 73.7 % vs. 72.2 %, p < 0.001). The overall survival (OS) at 5 years was significantly higher in group A than in groups B and C (90.1 vs. 84.4 % vs. 83.4 %, p < 0.001). However, there is no significant difference for RFS and OS between groups B and C (all, p > 0.05).
Conclusions
Decline of elevated CEA level (>5 ng/mL) during preoperative chemoradiotherapy has no significant effect on tumor response and recurrence in rectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preoperative chemoradiotherapy (CRT) followed by curative resection is the standard treatment for patients with locally advanced rectal cancer [1, 2]. In several studies, good and complete pathologic response after preoperative CRT has led to good prognosis in rectal cancer [3–5].
Serum carcinoembryonic antigen (CEA) is the generally used tumor marker in patients with colorectal malignancy. Initially elevated CEA level before treatment is informative for predicting poor prognosis of a colorectal cancer patient, and serial checkups of post-treatment CEA level are performed to detect tumor recurrence after curative surgery [6–9]. Normalization of the postoperative CEA level after curative surgery has been associated with improved recurrence-free survival (RFS) and overall survival (OS) in rectal cancer [10–12]. Several recent studies reported that an elevation of pre-CRT CEA (> 5 ng/mL) level is a negative predictor of tumor downstaging and reduction of CEA level after preoperative CRT in rectal cancer could be a favorable prognostic factor [11–13]. However, the optimal cutoff value of post-CRT CEA level and clinical consequence of CEA change during CRT for tumor response and recurrence have not yet been clearly settled in rectal cancer. In a retrospective study, a decrease of CEA level after CRT was an independent predictor of good tumor regression [13]. However, the study had some limitations for short-term follow-up, small number of enrolled patients, and the retrospective and single-center design.
Thus, it is worthwhile to evaluate whether the change of the CEA level between before and after CRT is related to tumor response and recurrence. We performed a retrospective multicenter study to analyze the significance of post-CRT CEA level for pathologic complete response after CRT and RFS.
Materials and methods
Patients
The multicenter retrospective study assessed the association between post-CRT CEA level and tumor response and recurrence. The study was carried out by the Korean Radiation Oncology Group (KROG). Eligibility criteria included histopathologically confirmed adenocarcinoma within 10 cm of the anal verge, clinical T3-4N0-2M0, and no history of previous malignancy. We enrolled 1804 patients with rectal cancer who underwent preoperative CRT and curative surgery between March 2003 and June 2014 at eight institutions in Korea. In the data analysis, 336 patients had no record of post-CRT CEA level before radical surgery, one patient had previous malignancy history, and 20 patients had unavailable pathologic reports. Thus, they were excluded and the remaining 1447 patients were finally analyzed for this study. Institutional review board approvals of all participating centers were obtained, and the patient data were transferred to the KROG Data Management Center.
Evaluation
Staging workups included digital rectal examination, flexible sigmoidoscopy, chest and pelvic computed tomography (CT) scans, pelvic magnetic resonance (MR) scan, and CEA measurements. Preoperative CEA levels have been checked before and after CRT. Clinical and pathologic stages were determined according to the American Joint Committee on Cancer Staging, 7th edition. Colorectal pathologists who were highly specialized evaluated the pathologic specimens and assessed the treatment response of preoperative CRT. A pathologic complete response (ypCR) definition was no viable tumor cells and only fibrotic mass in the pathologic specimen (ypT0N0).
Treatment
All patients received preoperative radiation of 50.4 Gy in 28 fractions. The radiotherapy consisted of whole pelvic radiotherapy dose of 45 Gy in 25 fractions and boost dose of 5.4 Gy in three fractions on the primary tumor. Concurrent chemotherapy consisting of bolus injection of 5-fluorouracil (400 mg/m2/day) and leucovorin (20 mg/m2/day) for the first and last week of radiotherapy or continuous injection of 5-FU (225 mg/m2/day) or oral capecitabine (825 mg/m2, twice daily) was administered during radiotherapy. Total mesorectal excision (TME) was performed 4 to 8 weeks after preoperative CRT.
Statistical analysis
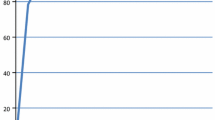
A receiver operating characteristic (ROC) analysis was performed to determine the optimal cutoff value of post-CRT CEA level for pathologic complete response after CRT. The threshold for maximum of (sensitivity + specificity) was calculated using ROC curve. The maximum value of the index (sensitivity + specificity) was represented as a criterion for selecting the optimal cutoff point [14]. Thus, post-CRT CEA point of 2.5 (area under curve, 0.593, p < 0.001) was adopted as the cutoff value in this study (Fig. 1). CEA level exhibited a maximum value of sensitivity (46.7 %) plus specificity (67.0 %) at 2.5 ng/mL. All predictive factors which were related to tumor response after CRT were analyzed by chi-square test. A logistic regression model was used in the multivariate analysis. RFS was defined as the time interval from the day of surgery to the day of any recurrence or death. OS was defined as the time interval from the day of surgery to the day of any death. The survival rate was estimated by the Kaplan-Meier method and compared by the log-rank test. A p value <0.05 by two-tailed tests was considered statistically significant. All statistical analyses were performed using SPSS version 12.0 (Chicago, IL, USA).
Results
Patient and tumor characteristics are summarized in Table 1. The study population was mostly male patients (69.0 %) with a median age of 62 years (range 27–84). The median distance between tumor margin and anal verge was 5 cm (range, 0–10). A total of 1360 (94.0 %) of 1447 patients had clinical T3 disease and 87 patients (6.0 %) had clinical T4 disease. 1144 (79.1 %) of 1447 patients had clinically positive node.
Tumor response after chemoradiotherapy
One hundred and sixty (11.1 %) of 1447 patients had ypCR after preoperative CRT. Tumor downstaging was observed in 707 (48.9 %) of 1447 patients, and nodal downstaging was observed in 858 (59.3 %) of 1447 patients. Table 2 shows results of univariate and multivariate analyses of factors associated with ypCR after CRT. On the univariate analysis, pre-CRT CEA (p = 0.001), post-CRT CEA (p = 0.036), clinical T classification (p = 0.007), clinical N classification (p < 0.001), tumor location (p = 0.001), and tumor size (p < 0.001) were significantly associated with ypCR. Additional multivariate analysis revealed that pre-CRT CEA [odds ratio (OR), 0.507 and 95 % confidence interval (CI), 0.343–0.750; p = 0.001], clinical T classification (OR, 0.589 and 95 % CI, 0.419–0.826; p = 0.021), clinical N classification (OR, 0.642 and 95 % CI, 0.443–0.930; p = 0.001), tumor location (OR, 1.42 and 95 % CI, 1.10–1.84; p = 0.006), and tumor size (OR, 0.619 and 95 % CI, 0.439–0.872; p = 0.002) were independent predictors for ypCR of rectal tumors.
Recurrence and survival
After curative surgery, 1306 (90.2 %) of 1447 patients received 5-FU- or capecitabine-based adjuvant chemotherapy. After a median follow-up time of 51 months, the 5-year RFS rate was 79.3 % and the 5-year OS rate was 88.1 % for all patients. Locoregional recurrence occurred in 64 patients, and distant recurrence occurred in 224 patients during the follow-up period.
The 5-year RFS rate was significantly higher in the post-CRT CEA of ≤2.5 ng/mL group than in the post-CRT CEA of >2.5 ng/mL group (82.7 vs. 74.3 %, p < 0.001; Fig. 2a). Twenty-nine patients (3.4 %) in the post-CRT CEA of ≤2.5 ng/mL group and 35 patients (5.8 %) in the post-CRT CEA of >2.5 ng/mL group had local recurrences. The difference of locoregional recurrence rates between two groups was significant (p = 0.037). One hundred thirteen patients (13.3 %) in the post-CRT CEA of ≤2.5 ng/mL group and 111 patients (18.5 %) in the post-CRT CEA of >2.5 ng/mL group had distant recurrences. The difference of distant recurrence rates between two groups was significant (p = 0.008). The 5-year RFS rate was significantly higher in the ypCR group than in the non-ypCR group (97.3 vs. 76.9 %, p < 0.001; Fig. 2b). The 5-year OS rate was significantly higher in the post-CRT CEA of ≤2.5 ng/mL group than in the post-CRT CEA of >2.5 ng/mL group (89.1 vs. 86.6 %, p = 0.003; Fig. 2C). The 5-year OS rate was significantly higher in the ypCR group than in the non-ypCR group (96.5 vs. 87.0 %, p < 0.001; Fig. 2d).
Based on the pre- and post-CRT CEA level, we categorized patients into three groups. Group A comprised pre-CRT CEA ≤5 ng/mL. Group B comprised pre-CRT CEA >5 ng/mL and post-CRT CEA ≤2.5 ng/mL. Group C comprised pre-CRT CEA >5 ng/mL and post-CRT CEA >2.5 ng/mL. All patients who had comprised pre-CRT CEA ≤5 ng/mL had post-CRT CEA ≤2.5 ng/mL. The 5-year RFS rate was significantly higher in group A than in group B and C (82.6 vs. 73.7 vs. 72.2 %, p < 0.001; Fig. 3a). The 5-year OS rate was significantly higher in group A than in groups B and C (90.1 vs. 84.4 vs. 83.4 %, p < 0.001; Fig. 3b). However, the differences of RFS and OS rate between groups A and B were significantly larger than between groups B and C (all, p < 0.001). The differences of RFS (p = 0.326) and OS (p = 0.414) rate between group B and C were not statistically significant.
Discussion
In our multi-institutional analyses, 160 (11.1 %) of 1447 patients achieved a ypCR after CRT. This is very similar to the results achieved by other series where 8–12 % of the patients in the preoperative CRT group had a ypCR [1–3]. Serum CEA is a valuable prognostic marker in colorectal cancer patients [15, 16]. In the present study, an elevated CEA level of >5 ng/mL was significantly associated with poor tumor response, and our results were in accordance with those of Yoon SM et al. [17], which suggest that a pretreatment CEA level of >5 ng/mL is a useful predictor of poor tumor regression. Post-treatment serial CEA can also provide an opportunity to detect the recurrent tumor [18, 19]. National Comprehensive Cancer Network guidelines suggest CEA as a tumor marker for following up colorectal cancer patients [20]. Several studies described the correlation between pretreatment CEA level and tumor response. Lee et al. reported that pre-CRT elevation of CEA level predicts poor tumor response and frequent distant recurrence in rectal cancer [21]. Park et al. described that an elevated pretreatment CEA level was an independent predictor for pathologic tumor response to CRT [22].
The prognostic significance of post-CRT CEA level in rectal cancer has been evaluated in only few series [13, 23]. In a recent retrospective study, the post-CRT CEA ≤2.7 ng/mL was identified as a good prognostic factor for disease-free survival [13]. In that study, post-CRT CEA level was also identified as a predictor of tumor regression and tumor down-staging. Our results suggest that post-CRT CEA <2.5 ng/mL could predict low locoregional (p = 0.037) and distant recurrence (p = 0.008) rate in rectal cancer patients who receive preoperative CRT and curative surgery. However, in the present study, post-CRT CEA ≤2.5 ng/mL was not a significant predictor of ypCR after CRT on multivariate analysis. In our analyses, we assessed the ypCR after the CRT according to the clinical characteristics. On the multivariate analysis, the pre-CRT CEA level (p = 0.001), cT classification (p = 0.021), cN classification (p = 0.001), and tumor size (p = 0.002) were significant factors for ypCR. These results are similar to those of Yoon et al. [17] who reported that cT and cN classification, pretreatment CEA, and tumor volume were significantly associated with tumor downstaging after CRT in rectal cancer.
We evaluated the 5-year RFS rate according to combination of pre-CRT and post-CRT CEA levels (group A: pre-CRT CEA ≤5 ng/mL; group B: pre-CRT CEA >5 ng/mL and post-CRT CEA ≤2.5 ng/mL; and group C: pre-CRT CEA >5 ng/mL and post-CRT CEA >2.5 ng/mL). The RFS rates at 5 years were 82.6 % in group A, 73.7 % in group B, and 72.2 % in group C. The difference of RFS and OS rates between group A and B was significantly larger than between groups B and C (all, p < 0.001), and there was no significant difference of 5-year RFS and OS rate between group B and C (all, p > 0.05). In the multivariate analysis, pre-CRT CEA (p = 0.001) levels more significantly affected pathologic tumor response than post-CRT CEA levels (p = 0.095). In our study, we perform a ROC analysis to determine the optimal cutoff value of post-CRT CEA level for ypCR after CRT. Although we deduct a cutoff value of 2.5 ng/ml through the Youden index [maximum of (sensitivity + specificity)], the shape of ROC curve for post-CRT CEA level is regularly convex, not angled. Thus, not only AUC but also sensitivity and specificity are relatively low at any point of post-CRT CEA level. Pre-CRT CEA level could have more significant impact on tumor response and RFS than post-CRT CEA levels.
This study had several limitations. First, our study should be understood in view of the inherent biases of a retrospective study design [24]. However, we conducted a multicenter retrospective study that enrolled 1447 patients in eight institutions, and all patients received a standard radiotherapy of 50.4 Gy in 28 fractions followed by total mesorectal excision. Thus, the evaluation of predictive factors seems reliable for those treated with this modality, which is the standard treatment of locally advanced rectal cancer. Second, concurrent chemotherapy was not delivered by the same regimen and schedule. Thus, we categorized chemotherapy regimens into three types (continuous 5-FU, bolus 5-FU and leucovorin, and oral capecitabine) and verified that the type of concurrent regimen had no significant effect on pathologic tumor response.
Conclusion
CEA change during preoperative CRT in rectal cancer has no significant effect on tumor response and recurrence-free survival. Pre-CRT CEA level rather than post-CRT CEA is an independent predictor for tumor response and recurrence-free survival in rectal cancer patients treated with preoperative CRT followed by curative resection.
References
Roh MS, Colangelo LH, O’Connell MJ, et al. (2009) Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 27:5124–5130
Sauer R, Becker H, Hohenberger W, et al. (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740
Rödel C, Martus P, Papadoupolos T, et al. (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696
Suárez J, Vera R, Balén E, et al. (2008) Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Color Dis 10:563–568
Vecchio FM, Valentini V, Minsky BD, et al. (2005) The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 62:752–760
Berman JM, Cheung RJ, Weinberg DS (2000) Surveillance after colorectal cancer resection. Lancet 355:395–399
Chen CC, Yang SH, Lin JK, et al. (2005) Is it reasonable to add preoperative serum level of CEA and CA19-9 to staging for colorectal cancer? J Surg Res 124:169–174
Moureau-Zabotto L, Farnault B, de Chaisemartin C, et al. (2011) Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 80:483–491
Farnault B, Moureau-Zabotto L, de Chaisemartin C, et al. (2011) Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer and correlation of these factors with survival. Cancer Radiother 15:279–286
Slentz K, Senagore A, Hibbert J, et al. (1994) Can preoperative and postoperative CEA predict survival after colon cancer resection? Am Surg 60:528–531 discussion 31-2
Park YA, Lee KY, Kim NK, et al. (2006) Prognostic effect of perioperative change of serum carcinoembryonic antigen level: a useful tool for detection of systemic recurrence in rectal cancer. Ann Surg Oncol 13:645–650
Chung MJ, Chung SM, Kim JY, et al. (2013) Prognostic significance of serum carcinoembryonic antigen normalization on survival in rectal cancer treated with preoperative chemoradiation. Cancer Res Treat 45:186–192
Jang NY, Kang SB, Kim DW, et al. (2011) The role of carcinoembryonic antigen after neoadjuvant chemoradiotherapy in patients with rectal cancer. Dis Colon rectum 254:245–252
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Wolmark N, Fisher B, Wieand HS, et al. (1984) The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and bowel project) clinical trials. Ann Surg 199:375–382
Park YJ, Park KJ, Park JG, et al. (1999) Prognostic factors in 2230 Korean colorectal cancer patients: analysis of consecutively operated cases. World J Surg 23:721–726
Yoon SM, Kim DY, Kim TH, et al. (2007) Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 69:1167–1172
Locker GY, Hamilton S, Harris J, et al. (2006) ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 24:5313–5327
Moertel CG, Fleming TR, Macdonald JS, et al. (1993) An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 270:943–947
Bevers TB, Anderson BO, Bonaccio E, et al. (2009) NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. JNCCN 7:1060–1096
Lee JH, Kim DY, Kim SH, et al. (2015) Carcinoembryonic antigen has prognostic value for tumor downstaging and recurrence in rectal cancer after preoperative chemoradiotherapy and curative surgery: a multi-institutional and case-matched control study of KROG 14-12. Radiother Oncol 116:202–208
Park YA, Sohn SK, Seong J, et al. (2006) Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol 93:145–150
Perez RO, São Julião GP, Habr-Gama A, et al. (2009) The role of carcinoembriogenic antigen in predicting response and survival to neoadjuvant chemoradiotherapy for distal rectal cancer. Dis Colon rectum 52:1137–1143
Lee SW, Hwang TK, Hong SH, et al. (2014) Outcome of postoperative radiotherapy following radical prostatectomy: a single institutional experience. Radiat Oncol J 32:138–146
Acknowledgments
The authors wish to acknowledge the financial support of the St. Vincent’s Hospital, Research Institute of Medical Science. Mi Joo Chung and Taek Keun Nam equally contributed to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chung, M.J., Nam, T.K., Jeong, J.U. et al. Can serum dynamics of carcinoembryonic antigen level during neoadjuvant chemoradiotherapy in rectal cancer predict tumor response and recurrence? A multi-institutional retrospective study. Int J Colorectal Dis 31, 1595–1601 (2016). https://doi.org/10.1007/s00384-016-2629-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2629-z