Abstract
Purpose
Patient and technical factors influencing the postoperative infectious complications (ICs) after elective colorectal resections are satisfactorily described. However, the underlying disease-related factors have not been extensively evaluated. This study aimed to measure the effect of malignancy on postoperative surgical site and extra surgical site infections after elective colorectal resection.
Methods
This study is a bicentric retrospective matched pair study of prospectively gathered data. Between 2004 and 2013, 1104 consecutive patients underwent colorectal resection in two centers. Patients undergoing elective resection with supraperitoneal anastomosis for benign diseases (excluding inflammatory bowel disease) (group B, n = 305) were matched to randomly selected patients with malignancy (group M, n = 305). The matching variables were age, gender, American Society of Anesthesiologists (ASA) score, malnutrition, type of resection, and surgical approach. We compared the 30-day IC rates between patients with benign diseases (group B) and malignancy (group M). Multivariate logistic regression analysis was performed to identify the risk factors for ICs.
Results
Group M had a higher overall rate of IC (25.6 vs 16.1 %, P = 0.004) as well as a higher risk of extra surgical site infections (P = 0.007) and anastomotic leakage (P = 0.039). The independent risk factors for ICs were malignancy (odds ratio (OR) = 2.02; P = 0.002), age ≥70 years (OR = 1.73, P = 0.018), tobacco history (OR = 1.87; P = 0.030), and obesity (OR = 1.68; P = 0.039).
Conclusion
Malignancy, age, tobacco history, and obesity increase the risk of ICs after colorectal resection. Improvement of the modifiable risk factors, increased compliance with an enhanced recovery after surgery (ERAS) program in the overall population, and optimization of immune function in patients with malignancy should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal surgery has both a known morbidity (10 to 40 %) and mortality (0.2 to 2 %) rates, which is largely as a result of the postoperative infectious complications (ICs). ICs include surgical site infections (SSIs), which are the most common nosocomial infections in surgical patients [1], and extra SSI (E/SSI), which are commonly pulmonary and urinary tract infections [2]. Because ICs result in longer hospital stays, a delayed resumption of normal activity [3], and a decreased long-term survival in patients undergoing curative surgery for colorectal cancer [4], reducing ICs after colorectal surgery is a major public health issue.
Numerous studies have focused on identifying predictive factors for morbidity and mortality in colorectal surgery. Several patients’ related factors (advanced age, male gender, high American Society of Anesthesiologists (ASA) score, and malnutrition) and numerous intraoperative factors (surgical site contamination, duration of intervention, and blood loss) have been demonstrated to be predictive in colorectal surgery [5], while the laparoscopic approach has been suggested to reduce SSI.
The study of host- and disease-related factors is a promising area of research for predicting and preventing IC [6]. Because colectomies for cancer are rarely individualized and are usually analyzed with other diseases [6], the influence of neoplastic disease on the rates of postoperative IC has been poorly evaluated in clinical studies [6, 7]. However, neoplastic disease is known to cause immunosuppression through dysregulating lymphocyte function, which may predispose patients to IC development [6, 8]. We recently demonstrated, through a case matched study, that for patients undergoing resection for a colorectal cancer, advanced tumor stage, malnutrition, obesity, and resection by laparotomy increase the risk of infectious complications after colorectal resection [9]. However, it remains unknown whether malignancy alone favors the development of these complications. The primary aim of this case–controlled study was to investigate whether malignancy correlates with the occurrence of IC after colorectal resection.
Materials and methods
Patients
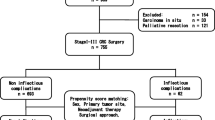
Between June 2004 and December 2013, 1104 consecutive patients underwent open or laparoscopic resection for colorectal cancer (CRC) or benign disease, either electively (n = 980) or as an emergency (n = 124), in our department of digestive surgery in Lille University Hospital, France, and in a private hospital, Clinique Mathilde, Rouen, France. All of these patients were included in a prospective evaluation exploring both the mortality and morbidity after colorectal surgery. Resections were performed for CRC (n = 742) or benign disease (n = 362), including diverticular disease, benign colorectal polyps, deep pelvic endometriosis, inflammatory bowel disease, colonic volvulus, stenosis secondary to chronic ischemia, and rare causes (redo-anastomosis for previous fistula or stenosis, colonic resection during postoperative hernia repair, and sigmoid resection during a rectopexy for prolapse).
The inclusion criteria for the present matched pair study were elective segmental resection with supra peritoneal anastomosis for CRC (n = 616) or benign disease (n = 305). Patients undergoing resection as an emergency (n = 124), resection for inflammatory bowel disease (n = 23), or rectal resection with infraperitoneal colorectal anastomosis or coloanal anastomosis were excluded (n = 46); also, rare causes (n = 8) were excluded (4 resections for redo-anastomosis and 4 colorectal resection in association with a mesh repair that may have biased analysis of postoperative IC). No patient underwent preoperative radiation.
A matched pair analysis using the frequency matching technique was constructed to test the hypothesis that malignancy favored postoperative IC. The study group (group B, n = 305) consisted of all patients fulfilling the inclusion criteria who underwent colorectal resection for a benign disease. According to the frequency matching technique, these patients were broadly matched 1:1 to randomly selected patients who underwent resection for a CRC and met the inclusion criteria during the same study period (group M, n = 305). Patients were matched according to major variables that have been reported to be linked with postoperative IC, including the age, sex, American Society of Anesthesiologists (ASA) grade, malnutrition (defined as weight loss of more than 10 % over a 6-month period), surgical approach (laparoscopy vs laparotomy), and type of resection [2, 10, 11]. Investigators were blinded to the operative outcome during the selection process.
Preoperative treatment and surgical approach
In the M group, patients’ cases were individually discussed during multidisciplinary meetings and treatment was decided according to the French national guidelines [12]. All patients received intravenous prophylactic antibiotics, and the type, timing, and duration of the antibiotic treatment were also decided according to French national guidelines [13]. Seven surgeons performed the colorectal resections. Preoperative bowel preparation was not administered. Povidone–iodine scrub was used for skin preparation in all patients. Colectomy was oncologic in group M. The operative technique and anastomotic technique were all performed at the discretion of the operating surgeon. Left colectomy included left hemicolectomy, superior segmental colectomy (resection of the descending colon and distal transverse colon with anastomosis between the remaining transverse and sigmoid colon), and sigmoid colectomy. Rectal resection included resection of the colorectal junction with a supraperitoneal colorectal anastomosis. Sepsis (infected tumor and/or abscess discovered during surgery) and intraoperative fecal soiling were systematically recorded. The use of closed suction drains and creation of a protective defunctioning stoma were based on the individual surgeons’ usual practices. Wound protection was standard during laparotomies, including during specimen extraction in laparoscopic procedures. Early mobilization, early resumption of diet, and nutritional supplements were systematically implemented. Otherwise, perioperative care was based on the individual surgeons’ usual practices. The 7th UICC/TNM classification was used for histopathological staining.
Variables studied
Data were collected from a prospectively maintained database. Obesity was defined by a body mass index ≥30 kg/m2 [14] and anemia was defined by a hemoglobin level <10 g dl−1 [15]. The blood loss and operative time were dichotomized using the 75th percentile as a threshold [16].
Group B patients were matched to group M patients according to the age, sex, ASA score, malnutrition, surgical approach, and type of resection; hence, preoperative parameters were comparable between the groups.
The primary endpoint was the IC rate within 30 days of surgery. ICs were defined according to the Centers for Disease Control and Prevention classifications by Horan et al. [17, 18] and consisted of the SSI and E/SSI.
The natures of SSIs were categorized as being incisional or organ space. Incisional SSIs were either superficial (involving only the skin and subcutaneous tissue) or deep (involving deep soft tissues, e.g., fascial and muscle layers). Organ space SSI concerned any part of the body, excluding the skin incision, fascia, or muscle layers, which was opened or manipulated during the operative procedure. Two groups of organ space SSI were identified: first, intra-abdominal abscesses in the absence of radiological or clinical evidence of an anastomotic leak and, second, intra-abdominal abscesses with radiological or clinical evidence of an anastomotic leak. E/SSI included symptomatic urinary, respiratory tract, hematological, or gastro-intestinal infections.
The secondary endpoints included (i) the 30-day overall morbidity, defined as any postoperative complication with a Dindo–Clavien score ≥1 [19]; (ii) 30-day major morbidity, including events requiring reinterventions (grade III in the Dindo–Clavien classification) and life-threatening complications (grade IV in the Dindo–Clavien classification) [19]; and (iii) in-hospital postoperative mortality.
Statistical analysis
Statistical analysis was performed using SPSS® version 15.0 software (SPSS, Chicago, IL). Data are shown as the prevalence or median (range). Continuous data were compared using the t test and ordinal data by the chi-square test or Fisher’s exact test, as appropriate. To determine predictors of IC, variables with P < 0.10 in univariable analysis were entered into a multivariable analysis using binary logistic regression analysis. All statistical tests were two-sided, and the threshold of significance was set at P < 0.05. The study complied with the French National Health guidelines on research involving human subjects.
Results
Composition of the study groups
In group B, the reasons for colorectal resection were the following: 188 with diverticular disease (61.7 %), 74 with benign colorectal polyps (including familial polyposis) (24.3 %), 27 with deep endometriosis and digestive involvement (8.9 %), 11 with colonic volvulus (3.6 %), and 5 with stenosis secondary to chronic ischemia (1.6 %).
In group M, the tumor location was the right colon in 79 cases (25.9 %), transverse colon in 11 cases (3.6 %), left colon in 207 cases (67.9 %), and supraperitoneal rectum in 8 cases (2.6 %). Histopathological analysis showed that 76 patients had pTNM stage I disease, 102 patients stage II (82 stage IIA and 20 stages IIB and IIC), 83 patients stage III (10 stage IIIA, 45 stage IIB, and 28 stage IIIC), and 44 patients stage IV disease.
Preoperative variables
The median patient age was 60.1 years (range 16.9–95.1) and the male-to-female ratio was 1.24:1. The patients’ ASA grade was I or II in 86.6 % of the cases (Table 1). Malnutrition affected 10.8 % of the patients and 20.2 % of patients were obese at the time of presentation. Most of patients underwent a left colectomy (65.4 %). A laparoscopic approach was attempted in 42 % of the patients. Matching variables were well balanced, as expected. The two groups were comparable in terms of the body mass index, alcohol and tobacco history, and neurologic comorbidity. However, diabetes mellitus and anemia were more frequent in group M (P = 0.006 and P < 0.001, respectively).
Intraoperative variables
In group B, infected or abscessed lesions and preoperative fecal soiling were more frequent (P < 0.001 and P = 0.001, respectively) (Table 2). In addition, the duration of the operation was longer in group B (P = 0.027). Other intraoperative variables (conversion, anastomotic characteristics, drain placement, and blood loss) did not differ significantly nor did the frequency with which an associated procedure was performed.
Primary endpoint
Details of the ICs are reported in Table 3. The IC rate at 30 days was 20.8 % and was significantly higher in group M than in group B (25.6 vs 16.1 %; P = 0.004). Anastomotic leaks accounted for 28.3 % of ICs and were significantly higher in group M than in group B (7.9 vs 3.9 %, P = 0.039). SSIs, incisional SSIs, and organ space SSIs did not significantly differ between the two groups (P > 0.258). The extra SSI rate was higher in group M than in group B (9.8 vs 4.3 %; P = 0.007).
Based on univariable analysis, in addition to group M (P = 0.004), six other variables were statistically related to IC as follows: age greater than 70 years (P = 0.039), obesity (P = 0.037), tobacco use history (P = 0.018), alcohol use history (P = 0.047), surgical approach (i.e., laparotomy; P ≤ 0.001), and protective stomia (P = 0.014) (Table 4). However, gender (P = 0.184), ASA score (P = 0.251), malnutrition (P = 0.172), diabetes mellitus (P = 0.131), neurologic comorbidity (P = 0.572), anemia (P = 0.252), conversion rate (P = 0.194), type of resection (P = 0.500), infected lesion (P = 0.483), fecal soiling (P = 0.101), type of anastomosis (P = 0.199), intra-abdominal drain placement (P = 0.115), blood loss (P = 0.650), transfusion (P = 0.376), and operative duration (P = 0.129) had no significant impact on the IC occurrence.
In the multivariable analysis, predictive factors of ICs were malignancy (P = 0.002), age greater than 70 years (P = 0.018), tobacco history (P = 0.030), and obesity (P = 0.039) (Table 5).
Secondary endpoints
The 30-day overall morbidity rate was 36.9 % (Table 3). Neither the 30-day overall morbidity nor the 30-day major morbidity (Clavien–Dindo grade III/IV complications) significantly differed between the groups (P = 0.154 and P = 0.385, respectively).
The in-hospital postoperative mortality rate was 1.5 % (P = 0.252). Peritonitis secondary to anastomotic leak was the primary cause of postoperative mortality in both groups M (n = 4) and B (n = 2). Other causes of postoperative mortality were mesenteric infarction (n = 1 in each group) and liver failure in a cirrhotic patient (n = 1) in group M.
Discussion
Both host- and disease-related factors are promising areas of research for predicting and preventing the postoperative morbidity [20]. Elective colectomies for cancer are rarely individualized and are usually either analyzed together or with other diseases or mixed with emergency surgery procedures [6]. We recently demonstrated that for patients undergoing colorectal resection for neoplasia, an advanced tumor stage increased the postoperative ICs [9]. In the present study, we investigated the impact of malignancy alone on the postoperative ICs in a large bicentric case-matched study. We have shown that ICs at 30 days are significantly increased in patients who undergo operations for malignancy compared to benign disease. Taking into account confounding factors, either by the matching technique for preoperative variables or by adjustment through the multivariable analysis for perioperative and postoperative variables, malignancy was identified as an independent predictive factor for ICs (odds ratio (OR) = 2.024, P = 0.002). Three other factors, age ≥70 years, obesity, and tobacco use, have already been described as predictive of increased ICs [20–23] and were confirmed as such in the current study.
The morbidity and IC rates that we report are comparable with previous studies [4, 9]. Despite a higher frequency of abscessed lesion and fecal soiling in group B, SSI, incisional SSI, and organ space SSI remained similar between the groups, suggesting a deleterious effect of malignancy on SSI and its different subcategories. The higher risk of ICs in group M was mainly due to a collective implication of extra SSI and anastomotic leakage. Extent of resection in CRC includes the whole mesocolon for lymphadenectomy reasons, which may impair blood supply of the anastomotic region and consequently explain this higher rate of anastomotic leakage. A recent meta-analysis did not find statistically significant differences between the high and low ligation of the inferior mesenteric artery in the anastomotic leak rate (OR = 1.02, 95 % CI 0.76–1.37) [24]. Anastomotic leak can, indeed, be caused by multiple factors (including host- and disease-related factors), but a significant role of the ligation location has not been identified yet.
To the best of our knowledge, this is the first dedicated comparative controlled study demonstrating the effect of malignancy on the occurrence of postoperative ICs. The impact of malignancy on the IC rates is significant because other factors that are well known to be related to IC that were tested, including the nutritional status and surgical approach (laparoscopy), and significant in univariate analysis were no longer significant in the multivariate analysis. In the specific population of patients undergoing colorectal resection for CRC, we previously demonstrated that predictive factors of IC, in addition to advanced tumor stage, included malnutrition, obesity, and resection by laparotomy [9]. Immunosuppression appears to be the common factor that is shared by all of the independent predictors of postoperative ICs that we identified. Alteration in the balance of lymphocyte subpopulations and reduction in the cytokine production in CRC, especially in advanced stages [25, 26], provides evidence for tumor-induced suppression of immune function. Open compared to laparoscopic resection has been associated with a longer disruption of the immunological homeostasis after CRC resection, mainly in the early stages [27]. Malnutrition and obesity are also known to reduce the cellular and humoral immune responses [28, 29]. Finally, it is well known that smokers have impaired immune function, leading to an increased risk in the postoperative ICs [30].
These results raise the question of what corrective actions in the patient’s history or perioperative care may be taken to minimize the postoperative ICs in colorectal resections, regardless of the indication, and more specifically for CRC.
Regarding obesity, it is self-evident that corrective dietary measures may be an option in benign cases, but they cannot be achieved in patients who present with a CRC. Paradoxically, these patients often present in a malnourished state despite their obesity, including sarcopenia, which has recently been showed to predict the postoperative complications after colorectal surgery, including ICs [31]. Recent studies suggest that using BMI to define obesity is suboptimal and that the waist circumference and waist/hip ratio are better predictive risk factors for morbidity and mortality after colorectal surgery [32]. A strict evaluation and correction of the nutritional status of obese patients may significantly improve the postoperative results.
Several recent studies have found that current smokers are at a significantly increased risk of postoperative morbidity, including ICs and mortality, after colorectal surgery [23, 33]. This finding persisted across malignant and benign diagnoses [23], and there was a reduction in the long-term overall survival in smokers who underwent an operation for a CRC [33]. A concerted effort should be made to promoting smoking cessation in all patients who are scheduled for elective colorectal surgery with smoking cessation of 4 or more weeks [23, 34]. However, delaying surgery for colorectal cancer for 4 weeks, with the concurrent risk of tumor growth, needs to be at least critically weighed against the potential benefits of smoking cessation.
Immunonutrition consists of a supplementation in the immuno nutrients, mainly arginine, omega-3 fatty acids, and nucleotides. A systematic short course of preoperative immunonutrition, regardless of the nutritional status, combined with a postoperative course in malnourished patients, has been shown to decrease both the postoperative infectious morbidity (incisional surgical site, extra surgical site, and organ space SSIs) and length of hospital stay through improving the immunometabolic host response, which has a potential positive impact on the long-term prognosis through modifying tumor lymphocyte infiltration [35, 36]. Recently, oral antibiotic bowel preparation has been shown to significantly reduce the SSI, length of stay, and number of readmissions in elective colorectal surgery [37]. Additionally, preoperative probiotics have been shown to decrease ICs with possible mechanisms attributed to the maintenance of the intestinal flora and restriction of bacterial translocation from the intestine. This may be representative of the enhancement of systemic/localized immunity and concurrent attenuation of the systemic stress response [38, 39].
Enhanced recovery after surgery (ERAS) is a perioperative and postoperative care concept that was initiated in the early 1990s with the aim of initially reducing the length of hospital stays following elective abdominal surgery. However, it is now well known that ERAS decreases postoperative complication rates after colorectal surgery compared with conventional postoperative care [40], and there is a potential attenuation of the immune cascade [41]. The LAFA trial reported the superiority of laparoscopic surgery over open surgery for colon cancer when both are combined with an ERAS program [42]. A recent study additionally supported the use of laparoscopic resection within an ERAS program as an independent factor associated with an improved outcome [43].
This study is limited by its retrospective nature, which may introduce bias. However, the bicentric nature of our investigation, which used prospective culling of variables with very limited missing data, strengthens our study. Moreover, the sample size and the combination of a matched pair design to a multivariate analysis provide sufficient statistical robustness. Of note, diabetes mellitus and anemia, two potential factors that may have impact the risk of IC, were more frequent in group M [44, 45]. Both had no significant impact on IC in univariate analysis meaning that the variables do not interfere with the conclusion that malignancy is a predictive factor of IC. On a statistical point of view, introducing too much variables in a matched study may lead to a deleterious impact on the robustness of the results while decreasing strongly the number of patients enrolled. We used ASA score to define existing comorbid conditions in the population, more than detail of each comorbidity to ensure a robust matching. Taking into account independently each comorbid factor may have had some confusions due to (i) no standardized definition of the severity of each complication and (ii) no data about the fact that such comorbid condition are stabilized or not.
We excluded rectal resections with infraperitoneal anastomosis, resections for inflammatory disease, resection for redo-anastomosis, and resections in association with use of non-absorbable mesh because of specificities, including radiation use, immunosuppressive therapies, and surgery that may otherwise introduce bias.
Conclusion
We have shown that malignancy is an independent risk factor for ICs after colorectal resection with other risk factors, including advanced age (≥70 years), obesity, and current smoking status. Optimization of the modifiable risk factors through strict evaluation and correction of the nutritional status and smoking cessation should be favored in an effort to reduce the ICs after CRC resection. Moreover, optimization of immune function in CRC patients and increased compliance with an ERAS program, including the use of laparoscopic surgery, should be considered.
References
Pendlimari R, Cima RR, Wolff BG, Pemberton JH, Huebner M (2012) Diagnoses influence surgical site infections (SSI) in colorectal surgery: a must consideration for SSI reporting programs? J Am Coll Surg 214:574–580. doi:10.1016/j.jamcollsurg.2011.12.023
Rovera F, Dionigi G, Boni L, Piscopo C, Masciocchi P, Alberio MG, Carcano G, Diurni M, Dionigi R (2007) Infectious complications in colorectal surgery. Surg Oncol 16(Suppl 1):S121–S124
Reddy KM, Meyer CE, Palazzo FF, Conaghan P, Blunt MC, Stebbings WS, Leicester RJ, Cullen PT (2003) Postoperative stay following colorectal surgery: a study of factors associated with prolonged hospital stay. Ann R Coll Surg Engl 85:111–114
Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH (2015) Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg 261:497–505. doi:10.1097/SLA.0000000000000854
Young H, Knepper B, Moore EE, Johnson JL, Mehler P, Price CS (2012) Surgical site infection after colon surgery: National Healthcare Safety Network risk factors and modeled rates compared with published risk factors and rates. J Am Coll Surg 214:852–859. doi:10.1016/j.jamcollsurg.2012.01.041
Piessen G, Muscari F, Rivkine E, Sbaï-Idrissi MS, Lorimier G, Fingerhut A, Dziri C, Hay JM, FRENCH (Fédération de Recherche EN CHirurgie) (2011) Prevalence of and risk factors for morbidity after elective left colectomy: cancer vs noncomplicated diverticular disease. Arch Surg 146:1149–1155. doi:10.1001/archsurg.2011.231
Biondo S, Kreisler E, Fraccalvieri D, Basany EE, Codina-Cazador A, Ortiz H (2012) Risk factors for surgical site infection after elective resection for rectal cancer. A multivariate analysis on 2131 patients. Color Dis 14:e95–e102. doi:10.1111/j.1463-1318.2011.02798.x
Ishikawa M, Nishioka M, Hanaki N, Miyauchi T, Kashiwagi Y, Ioki H, Kagawa A, Nakamura Y (2009) Perioperative immune responses in cancer patients undergoing digestive surgeries. World J Surg Oncol 7:7. doi:10.1186/1477-7819-7-7
Bot J, Piessen G, Robb WB, Roger V, Mariette C (2013) Advanced tumor stage is an independent risk factor of postoperative infectious complications after colorectal surgery: arguments from a case-matched series. Dis Colon Rectum 56:568–576. doi:10.1097/DCR.0b013e318282e790
Suding P, Jensen E, Abramson MA, Itani K, Wilson SE (2008) Definitive risk factors for anastomotic leaks in elective open colorectal resection. Arch Surg 143:907–11. doi:10.1001/archsurg.143.9.907
Poon JT, Law WL, Wong IW, Ching PT, Wong LM, Fan JK, Lo OS (2009) Impact of laparoscopic colorectal resection on surgical site infection. Ann Surg 249:77–81. doi:10.1097/SLA.0b013e31819279e3
Thesaurus National de Cancérologie Digestive– chapitre 3: Cancer du colon. http://www.TNCD.org. Accessed 22 Dec 2015
Société française d’anesthésie et de réanimation (2011) Antibioprophylaxis in surgery and interventional medicine (adult patients). Actualization 2010. Ann Fr Anesth Reanim 30:168–190. doi:10.1016/j.annfar.2010.05.012
Gendall KA, Raniga S, Kennedy R, Frizelle FA (2007) The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum 50:2223–2237
Tekkis PP, Prytherch DR, Kocher HM, Senapati A, Poloniecki JD, Stamatakis JD, Windsor AC (2004) Development of a dedicated risk-adjustment scoring system for colorectal surgery (colorectal POSSUM). Br J Surg 91:1174–1182
de Oliveira AC, Ciosak SI, Ferraz EM, Grinbaum RS (2006) Surgical site infection in patients submitted to digestive surgery: risk prediction and the NNIS risk index. Am J Infect Control 34:201–207
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20:271–274
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K, Association Française de Chirurgie (2005) Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg 140:278–283
Gervaz P, Bandiera-Clerc C, Buchs NC, Eisenring MC, Troillet N, Perneger T, Harbarth S (2012) Scoring system to predict the risk of surgical-site infection after colorectal resection. Br J Surg 99:589–595. doi:10.1002/bjs.8656
Grønkjær M, Eliasen M, Skov-Ettrup LS, Tolstrup JS, Christiansen AH, Mikkelsen SS, Becker U, Flensborg-Madsen T (2014) Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg 259:52–71. doi:10.1097/SLA.0b013e3182911913
Sharma A, Deeb AP, Iannuzzi JC, Rickles AS, Monson JR, Fleming FJ (2013) Tobacco smoking and postoperative outcomes after colorectal surgery. Ann Surg 258:296–300. doi:10.1097/SLA.0b013e3182708cc5
Cirocchi R, Trastulli S, Farinella E, Desiderio J, Vettoretto N, Parisi A, Boselli C, Noya G (2012) High tie versus low tie of the inferior mesenteric artery in colorectal cancer: a RCT is needed. Surg Oncol 21:e111–e123. doi:10.1016/j.suronc.2012.04.004
Matsuda A, Furukawa K, Suzuki H, Kan H, Tsuruta H, Matsumoto S, Shinji S, Tajiri T (2007) Does impaired TH1/TH2 balance cause postoperative infectious complications in colorectal cancer surgery? J Surg Res 139:15–21
Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG (2000) Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer 82:1009–1012
Ferri M, Rossi Del Monte S, Salerno G, Bocchetti T, Angeletti S, Malisan F, Cardelli P, Ziparo V, Torrisi MR, Visco V (2013) Recovery of immunological homeostasis positively correlates both with early stages of right-colorectal cancer and laparoscopic surgery. PLoS One 8:e74455. doi:10.1371/journal.pone.0074455
Donohoe CL, Feeney C, Carey MF, Reynolds JV (2011) Perioperative evaluation of the obese patient. J Clin Anesth 23:575–586. doi:10.1016/j.jclinane.2011.06.005
Braga M, Vignali A, Zuliani W, Radaelli G, Gianotti L, Martani C, Toussoun G, Di Carlo V (2002) Metabolic and functional results after laparoscopic colorectal surgery: a randomized, controlled trial. Dis Colon Rectum 45:1070–1077
Hersey P, Prendergast D, Edwards A (2003) Effects of cigarette smoking on the immune system. Follow-up studies in normal subjects after cessation of smoking. Med J Aust 2:425–429
Huang DD, Wang SL, Zhuang CL, Zheng BS, Lu JX, Chen FF, Zhou CJ, Shen X, Yu Z (2015) Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after colorectal cancer surgery. Color Dis. doi:10.1111/codi.13067
Kartheuser AH, Leonard DF, Penninckx F, Paterson HM, Brandt D, Remue C, Bugli C, Dozois E, Mortensen N, Ris F, Tiret E, Waist Circumference Study Group (2013) Waist Circumference Study Group. Waist circumference and waist/hip ratio are better predictive risk factors for mortality and morbidity after colorectal surgery than body mass index and body surface area. Ann Surg 258:722–730. doi:10.1097/SLA.0b013e3182a6605a
Richards CH, Platt JJ, Anderson JH, McKee RF, Horgan PG, McMillan DC (2011) The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Ann Surg 254:83–89. doi:10.1097/SLA.0b013e31821fd469
Mills E, Eyawo O, Lockhart I, Kelly S, Wu P, Ebbert JO (2011) Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med 124:144–154.e8. doi:10.1016/j.amjmed.2010.09.013
Kirk HJ, Heys SD (2003) Immunonutrition. Br J Surg 90:1459–1460
Caglayan K, Oner I, Gunerhan Y, Ata P, Koksal N, Ozkara S (2012) The impact of preoperative immunonutrition and other nutrition models on tumor infiltrative lymphocytes in colorectal cancer patients. Am J Surg 204:416–421. doi:10.1016/j.amjsurg.2011.12.018
Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT (2015) Oral antibiotic bowel preparation significantly reduces surgical site infection rates and readmission rates in elective colorectal surgery. Ann Surg 262:331–337. doi:10.1097/SLA.0000000000001041
Zhang JW, Du P, Gao J, Yang BR, Fang WJ, Ying CM (2012) Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am J Med Sci 343:199–205. doi:10.1097/MAJ.0b013e31823aace6
Liu ZH, Huang MJ, Zhang XW, Wang L, Huang NQ, Peng H, Lan P, Peng JS, Yang Z, Xia Y, Liu WJ, Yang J, Qin HL, Wang JP (2013) The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr 97:117–126. doi:10.3945/ajcn.112.040949
Spanjersberg WR, Reurings J, Keus F, vanLaarhoven CJ (2011) Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 16:CD007635. doi:10.1002/14651858.CD007635.pub2
Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, Zhong Y, Xue Z, Jin L, Zhan S, Niu W, Qin X, Wu Z, Wu Z (2012) Enhanced recovery after surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg 36:407–414. doi:10.1007/s00268-011-1348-4
Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA, Sprangers MA, Cuesta MA, Bemelman WA, LAFA study group (2011) Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 254:868–875. doi:10.1097/SLA.0b013e31821fd1ce
ERAS Compliance Group (2015) The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 261:1153–1159. doi:10.1097/SLA.0000000000001029
Lin X, Li J, Chen W, Wei F, Ying M, Wei W, Xie X (2015) Diabetes and risk of anastomotic leakage after gastrointestinal surgery. J Surg Res 196:294–301. doi:10.1016/j.jss.2015.03.017
Iancu C, Mocan LC, Todea-Iancu D, Mocan T, Acalovschi I, Ionescu D, Zaharie FV, Osian G, Puia CI, Muntean V (2008) Host-related predictive factors for anastomotic leakage following large bowel resections for colorectal cancer. J Gastrointestin Liver Dis 17:299–303
Acknowledgments
The authors would like to thank Dr. Guillaume Taillier, Dr. Thomas Fourrure, and Dr. Nadia Boubchir for the help in creating and maintaining the database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Sources of funding
None.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration as well as its later amendments or comparable ethical standards. Because this is a retrospective study, formal informed consent was not required.
Additional information
Thibault Crombe and Jérôme Bot contributed equally to this work.
Rights and permissions
About this article
Cite this article
Crombe, T., Bot, J., Messager, M. et al. Malignancy is a risk factor for postoperative infectious complications after elective colorectal resection. Int J Colorectal Dis 31, 885–894 (2016). https://doi.org/10.1007/s00384-016-2521-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2521-x