Abstract
Introduction
X-ray defecography is considered the gold standard for imaging pelvic floor pathology. However, it is limited by the capability to demonstrate only the posterior pelvic compartment, significant radiation exposure, and inconvenience. Dynamic transperineal ultrasound (DTP-US) can visualize all of three pelvic floor compartments, is free of radiation, and does not cause significant discomfort. The aim of this study was to evaluate the level of consistency between defecography (DEF) and DTP-US in the diagnosis of pelvic floor deformations.
Methods
One hundred and five women (age 56 ± 11 years) suffering from constipation and fecal incontinence were clinically evaluated and further examined by DEF and DTP-US. The rate of diagnosis of pelvic floor hernias using the DTP-US was compared to that found on DEF.
Results
The specificity for the diagnosis of rectoceles was of 82 % for mid-size rectocele and 98 % for large rectoceles, and the sensitivity was of 59 % for mid-size rectoceles and 50 % for larger rectoceles. The sensitivity for the detection of intussusceptions, enteroceles, and rectal prolapse were 82, 74, and 75 %, respectively. The specificity was 84 % for the detection of intussusception, 92 % for enteroceles, and 97 % for the diagnosis of rectal prolapse. Higher rates of DTP-US diagnosis were obtained when the intussuscepted rectum moved closer toward the ultrasound probe.
Conclusions
The sensitivity of DTP-US was good to excellent and the specificity was high. The added value of this technique in exploring all the compartments of the pelvic floor as well as the perineal muscles makes DTP-US a preferred procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The reported prevalence of pelvic floor disorders (PFD) in western female population is 12–20 % [1, 2]. In the recent years, better pathophysiological understanding of PFD has led to the development of an integrative multi-disciplinary approach. Combined teams of committed gastroenterologists, surgeons, gyneco-urologists, and uro-gynecologists were created to treat these complex disorders.
Dynamic imaging of the entire pelvic floor has a significant role in the investigation of patients with PFD. The ideal technique should enable the visualization of anatomical pelvic deformations at strain as well as diagnose pelvic organ prolapse (POP) in all three pelvic compartments during a single examination, with no radiation and a minimal level of inconvenience. X-ray defecography (DEF) with opacification of the small bowel and vagina is still considered as the gold standard diagnostic procedure for posterior compartment disorders [3]. However, it is limited by a significant radiation exposure and by the reports of significant inconvenience during the exam [4, 5]. Moreover, DEF is restricted to the demonstration of the posterior pelvic compartment. This limited field of investigation by DEF restrains its use for the understanding of the global nature of pelvic floor disorders. Dynamic cystocolpoproctography (DCP) was described by Kevin in 1994 [6] and modified later by Maglinte in 2011 [7]. Although DCP enables the examination of all three pelvic compartments, bladder catheterization is required in order to introduce contrast material. Therefore, DCP is invasive, inconvenient, and requires significant exposure to radiation.
Advanced techniques for dynamic pelvic imaging have been developed in the recent years. These techniques include dynamic magnetic resonance (MR) defecography [8], dynamic transperineal ultrasound (DTP-US) [9], and dynamic anal endosonography [10]. Dynamic MR defecography is limited by its high cost and low availability, while dynamic anal endosonography is restricted for the diagnosis of posterior compartment disorders. Dynamic transperineal ultrasound enables investigation of all three compartments of the pelvic floor with a standard two dimensional ultrasound device, is free of radiation, and does not cause significant discomfort to the patients.
Recent studies reported good level of consistency between DEF and DTP-US [4, 9, 11]. However, most of these studies were limited by a small number of participants and were restricted solely to the investigation of pathologies in the posterior compartment of patients with obstructed defecation.
This study was designed to assess the level of consistency between DEF and DTP-US in the diagnosis of pelvic floor deformations. We investigated a large heterogeneous cohort of women with chronic constipation and fecal incontinence and report the associated anatomical deformations in all three pelvic compartments.
Methods
Patients
This study is a retrospective review of data that had been collected in a population of adult females that were referred to our clinic for the evaluation of evacuation disorders (chronic constipation and fecal incontinence) during the years 2011 to 2013. As part of their evaluation, all patients filled a comprehensive clinical questionnaire targeted for the diagnosis and to the assessment of the severity of constipation, fecal incontinence, and irritable bowel syndrome. We used the bowel component of the Birmingham Bowel and Urinary Symptoms Questionnaire-22 for evacuation disorders, the Cleveland Constipation Severity Index (SCCI) scoring system for the assessment of constipation severity [12], the Wexner scoring system for fecal incontinence [13]. As part of their evaluation, all patients were examined by DTP-US and DEF.
Clinical parameters and definitions
Chronic constipation was defined as two of three symptoms such as straining, hard stool, unproductive call, infrequent stools for less than 3 weeks, or incomplete evacuation 25 % of the time. Symptoms duration was defined as at least 3 months since onset and beginning at least 6 months prior to diagnosis [14]. Fecal incontinence was defined as recurrent uncontrolled passage of fecal material for at least 3 months [14]. Symptom severity for fecal incontinence was determined based on the Wexner score [13]. In order to include only patients with significant symptoms, we included in the fecal incontinence group only patients who had solid or liquid fecal incontinence more than once a month. We assessed chronic constipation symptoms based on the constipation scoring system for the assessment of constipation severity [12]. We included in the constipation group only patients who scored 15 or higher.
Dynamic transperineal ultrasound
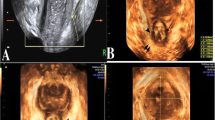
DTP-US was previously described by our group [9]. DTP-US is readily performed after rectal cleansing with one enema. DTP-US was conducted using a curvilinear 5–8 MHz (B&K, Profocus Ultra View, Herlev, Denmark) probe after liberal application of acoustic gel to the perineum and instilling 10 ml gel intravaginally and 50 ml gel into the rectum. The examination was then performed while the patient was lying in the left lateral position at rest, at squeeze, and at straining. Anal examination was performed with the transducer initially applied transversely to the perineal body in order to identify the axial view of the anus using as the landmark the hypo echoic ring of the internal anal sphincter. The transducer was then turned 180° to obtain a sagittal view of the contrast-filled rectum with extension of the hypoechoic internal anal sphincter appearing above and below the anal canal in profile. The anorectal junction was well seen with the bright hyperechoic elliptical bundle of the puborectalis sling demonstrable in relief. The anal canal, already identified in the initial US sweep for landmarks, was examined in more detail during forcible straining and simulated evacuation of the intra-rectal acoustic gel. The small bowel was easily defined by its motility. Definitive diagnoses may be made of a rectocele, intussusception, enterocele, or cystocele, with determination of rectocele depth and cystocele grade. Enterocele was diagnosed in our patients if a herniation of the abdominal contents developed anterior to the anorectal junction and extended into the vagina through cul-de-sac [15]. Rectocele was diagnosed by an anterior bulging of the rectum into the posterior vaginal wall. Rectocele depth was measured perpendicular to a line projected along the expected contour of the anterior rectal wall. We included in this study only significant rectoceles with a depth of at least 2 cm or more. Rectoceles of 2–4 cm in depth were considered mid-sized while those deeper than 4 cm were considered large. Intussusception was defined as folding of the rectum into either the rectum (recto-rectal) or in contact with the anus (recto-anal) or penetrating to the anal canal (intra-anal). Cystocele was defined as a herniation of the bladder into the anterior vaginal wall. We included in this study only significant cystoceles (≥grade 2) observed when the bladder sinks far enough to come close to the opening of the vagina. Anismus was defined as a closure or non-opening of the posterior anorectal angle during strain, which is a function of the contraction of the puborectalis muscle. Perineal descent was defined as a pelvic floor descent of more than 2 cm. All exams were performed and interpreted by a single physician (MBG) who was blinded to the results of the DEF.
Defecography
Evacuation proctography was performed without prior bowel preparation with 120 ml barium paste (55 % wt/wt barium sulfate) instilled into the rectum using conventional videofluoroscopy while the patient was in the lateral sitting position at rest and during evacuation in accordance with the basic technique described previously [16]. Quantitative measurements were taken during defecography for comparison with the DTP-US technique. The anorectal angle (ARA) was measured from the proctographic film at the junction of the axis of the anal canal and the rectal ampulla. Movement of the anorectal junction (ARJ) was assessed in relation to a horizontal line drawn across the most inferior point of the ischial tuberosities visible on lateral films. A rectocele was defined as any outpouching of the anterior rectal wall occurring during evacuation or straining. Rectoceles were assigned to a one of two groups based on depth. Mid-sized rectoceles were 2–4 cm deep and large rectoceles were defined as deeper than 4 cm. Enterocele was diagnosed and graded in the same way as was described for DTP-US. When recto-anal intussusception was identified, it was graded as described in the DTP-US section [16]. The small bowel was opacified following ingestion of 100 ml dilute oral diatrizoate meglumine (Gastrografin, Schering UK) 40 min prior to the examination. All proctographic examinations and measurements were done by a single examiner who was blind to the results of the DTP-US.
Statistical analysis
DEF was considered to be the gold standard technique for the calculation of sensitivity, specificity, the positive and negative predictive values, and the agreement of detection various prolapses with DPT-US in comparison with DEF. Agreement is the proportions of true results obtained by DTP-US in all the patients that had DEF performed. Statistical analysis was performed using the SPSS software (16.0 version; SSPS INC; Chicago, IL. USA). The χ 2 test or Fisher exact test was used to determine whether there was a significant association between the technique chosen and the number of pathologies found. Further subanalysis was performed according to the patient’s main complaint (constipation or fecal incontinence). Results were considered statistically significant when p ≤ 0.05. Accuracy is the proportions of true results obtained by DTP-US, either true positive or true negative, in the population of the study. It measures the degree of veracity of a diagnostic obtain by DTP-US.
Results
One hundred and five women were included in this study. The mean age of the cohort was 56 ± 11 years (29–80), and the mean parity was 2.8 ± 1.2. Eighty one patients were evaluated for chronic constipation (mean age 54.6 ± 11 years), and 24 for fecal incontinence (mean age 60 ± 12 years).
DTP-US revealed a mid-size rectocele in 32/105 (30 %), a large rectocele in 8/105 (8 %), an enterocele in 22/105 patients (21 %), an intussusception in 45/105 (43 %), and a rectal prolapse in 10/105 patients (9 %). In the same group, DEF revealed 34 mid-size rectocele (33 %), 12 large rectoceles (11 %), 23 enteroceles (22 %), 44 intussusceptions (42 %), and 8 rectal prolapses (8 %). The comparison of the anatomical abnormalities diagnosed in DEP and DTP-US in the whole cohort is summarized in Table 1. With DTP-US, the specificity for the diagnosis of rectoceles was of 82 % for mid-size rectocele and 98 % for large rectoceles. The sensitivity for the detection of rectoceles by DTP-US was lower: 59 % for mid-size rectoceles and 50 % for larger rectoceles. For mid-size rectoceles, the rate of false negatives was 26 % (12/46) for DTP-US and 30 % (14/46) for DEF. Larger rectoceles were missed by DEF in 43 % of the cases and 14 % of cases by DTP-US (2/4). The sensitivity and specificity for the detection of intussusceptions, enteroceles, and rectal prolapse with the two imaging techniques was high, with good sensitivity and high specificity. The sensitivity rates were 82, 74, and 75 %, respectively. The specificity was even higher: 84 % for the detection of intussusception, 92 % for enteroceles, and 97 % for the diagnosis of rectal prolapse. Higher rates of DTP-US diagnosis were obtained when the intussuscepted rectum moved closer toward the ultrasound probe. Recto-rectal intussusceptions were diagnosed by DEF in 27 cases versus 17 with DTP-US. Intra-anal intussusception was more commonly observed by DTP-US (7 DTP-US versus 4 with DEF). Larger enteroceles (grade 2 at least) were observed more commonly by DTP-US. Nine patients had grade 2 enterocele with DTP-US versus 1 with DEF. Anatomical pathologies in the anterior and middle compartments were detected solely by DTP-US. The pathologies included uterine prolapse in 8 cases, significant bladder neck descent (>2 cm) in 71 patients, and cystoceles in 53 patients (grade 1 in 25 cases, grade 2 in 26 cases, and grade 3 in 3 cases). Internal anal sphincter tear was diagnosed in 9 patients, external anal tear in 2 patients, and combined internal and external tears in one patient. These anal defects were further confirmed by endoanal ultrasound.
The comparison of the pelvic floor anatomical abnormalities diagnosed by DEF and DTP-US in constipated patients is summarized in Table 2. Similar to the previous reported results, the specificity for the detection of intussusception, enteroceles, and rectal prolapses was high: 85, 97, and 100 %, respectively. The specificity for the diagnosis of rectocele was high for mid-size as well as for large rectocele (84 and 96 %, respectively). The sensitivity was moderate except for intussusception which was high (91 %). Table 3 summarizes the comparison of the pelvic floor anatomical abnormalities between DEF and DTP-US in the fecal incontinence group. The sensitivity of rectoceles detection, rectal prolapse, and anismus was moderate to high (50 to 100 %), and the specificity of the diagnosis of intussusception, enterocele, and rectal prolapse was high as well (77–91 %).
Discussion
Pelvic floor hernias (rectoceles, enteroceles, cystoceles) are a significant cause of morbidity and reduction in quality of life. They often share the same risks factors and are related to elevation of abdominal pressure [17]. Different pelvic hernias may coexist regardless of their distribution in the pelvic floor. The occurrence of these hernias is also related to the association of these organs to the pelvic muscles function and with the quality of their fixation to the fascia. These frequent and multi-compartmental deformations require a dynamic pelvic diagnostic imaging method that is able to examine all the pelvic segments. DTP-US is a low cost, non-ionizing diagnostic method, which allows the simultaneous evaluation of all the pelvic structures by the specialists in charge of the treatment. Therefore, it was important to compare this method to DEF which is considered to be the gold standard imaging technique for the diagnosis of pelvic floor dynamic deformations at strain.
The results of our study demonstrate a good agreement between DEF and DTP-US for the detection of posterior pelvic floor dysfunctions at strain in patients suffering from any kind of defecation disorders. In the present study, a very high accuracy of DTP-US was found for the detection of large rectocele, enterocele, intussusception, and rectal prolapse (92, 89, 83, 94 %, respectively). The level of concordance was good for the diagnosis of mid-size rectoceles (74 %). Higher rates of DTP-US diagnosis were obtained when the intussuscepted rectum moved closer toward the ultrasound probe. Only DTP-US could provide information on the anterior pelvic compartment.
Neither DTP-US nor DEF can provide a completely accurate image of the defecation process. The pathologies identified by these methods are diagnosed in a non-physiological way. There are dependent upon the method used, the environmental conditions during the study and the cooperation of the examinee. During DEF or DTP-US, the examinee does not feel a spontaneous need to defecate, and therefore, the results of the study may be predisposed to his understanding, his active participation, and the intensity of the produced straining effort. The differences in the results of DEF and DTP-US may be explained by the differences between the two methods. DTP-US and DEF vary in the quality and the quantity of the contrast material, the position of the patient during the study, and the setting of the procedure. The force of gravity that is related to the different position during the procedure may also affect the study, as the weight of an organ changes according to its filling, and may interfere inversely with the pelvic floor. DEF has the advantage of placing the patient in a conventional sitting position, which is more acceptable and enables an easier rectal evacuation. As DTP-US is not a standardized technique, the reported position of the patients during the study varies. In some studies, the examinees were reported to be in a supine position during the exam, possibly hampering the physiologic process of defecation. However, when the patient lies on his left side with his knees bent, as in the method we describe, the angulation between the femur and the pelvis is similar to a position sitting, which is more physiological. Moreover, the relaxation of the puborectalis muscle is greater when the knees are bent closer to the chest. The fact that DTP-US is not standardized may explain the alterations in the sensitivity and in the specificity of DTP-US observed in this study when compared to previously published data [18].
The use of different quantities of contrast materials as well as the different levels of rectal filling may also affect in the diagnostic sensitivity of DEF and DTP-US. In general, DEF utilizes about three times more intra-rectal contrast than DTP-US; therefore, compression of the rectovaginal space by over-distension of the rectum is possible. The sensation of rectal filling may help the process of rectal evacuation by consciously stimulating the patient to strain. When evacuation of the contrast is achieved, the levator ani and the anal sphincters are relaxed, enabling visualization of rectal wall structural changes. Evacuation of the rectum empties the mid-pelvic compartment and can unmask a prolapse of the surrounding organs, e.g., intussusception, enterocele, and rectal prolapse. Especially, enteroceles may become evident only when both the rectum and bladder are evacuated [19, 20], as a result of the diminishing space occupied by the distended rectum and urinary bladder. This enables the penetration of the cul-de-sac by small bowel loops. Bremmer and colleagues revealed that the instillation of more than 250 ml of barium into the rectum with distension of the viscera to >10 cm in diameter could diminish the yield of the diagnosis of enterocele by 50 % [21].
The focal range of the US probe may also impact the accuracy of DTP-US. For instance, more recto-rectal intussusceptions were diagnosed by DEF (27 with DEF versus 17 with DTP-US). On the other hand, intra-anal intussusceptions that were closer to the US probe during the exam were more commonly observed by DTP-US (7 DTP-US versus 4 with DEF). Larger enteroceles (grade 2) were diagnosed more frequently by DTP-US (9 grade 2 with DTP-US versus 1 with DEF), probably due to the same cause.
The specificity of detection of mid-size and large rectoceles using DTP-US was 82 and 98 %, respectively, and the accuracy of the diagnosis of rectocele was higher in comparison to prior publications [4, 22]. On the other hand, the sensitivity for the diagnosis of rectoceles was only fair. This discordance may be explained by the imaging modality technique, by the experience of the observer, and by the examinee’s cooperation. Variation in the patient’s position during the procedure may also provide a possible explanation. DTP-US may miss rectocele due to the lying position of the patient.
However, it is important to notice that the association of diagnosis of rectocele to the symptoms of constipation is not clear [23], and only large rectoceles, which are evacuated by vaginal splinting, are probably significant [24, 25].
The sensitivity and the specificity for the detection of enterocele, intussusception, and rectal prolapse were high (74, 94, 82 % for DEF and 84, 75, 95 % for DTP-US, respectively) in this study. Clinically, the differentiation between rectocele and enterocele might be difficult; however, it is essential when surgery is considered. In this case, the DTP-US is effective in the diagnosis of enterocele. As previously mentioned, these results may not only relate to the accuracy of the imaging methods but are also influenced by other variables, as the patient’s cooperation, his understanding of the examination, and the quality of bearing down and rectal evacuation of the contrast. In order to diminish the patient’s embarrassment (in order to enable the best evacuation of the rectum), we ask the examinee to clear the rectum prior to the DTP-US with the use of an enema so that the examinee will know that their rectum is empty. We usually show the patients, at the beginning of the ultrasound examination, that their rectum is full of the gel we injected and free of stool. Interestingly, in previous studies, DTP-US was preferred by the majority of the patients who was examined by both methods [4, 18]. This may suggest that the patients are more relaxed during the US examination. High prevalence of psychiatric disorders and emotional disturbance was demonstrated in constipated patients undergoing defecating proctography [26]. These various factors, influenced by patient behavior, can alter the rate of detection of PFH’s regardless of the examination performed.
DTP-US enables the demonstration of pathologies in the anterior and mid-pelvic floor compartments, in contrast to DEF [7, 27]. This substantially improves the clinical yield of the procedure, as up to 95 % of the patients that suffer from defecation disorders may have multi-compartment dysfunctions, particularly uro-gynecological pathology [28]. Although all pelvic compartments can be visualized using dynamic cystoproctography, bladder catheterization makes the examination much more invasive. Another advantage of DTP-US is the possibility of imaging the anal canal, enabling the diagnosis of anatomical pathologies like tears. Another significant benefit of DTP-US is the lack of radiation, especially when considering the relative high radiation dose (15 ± 5 mSv) during DEF [29].
Ideally, we should have repeated the DEF in order to confirm the sensitivity of DTP-US and to re-evaluate the false positive findings on DTP-US; however, due to obvious ethical causes, this could not have been done. Therefore, pathologies which were diagnosed during DTP-US were considered as false positive when not observed by DEF, as DEF is considered the gold standard. However, DEF received criticism over the years due to significant inter-observer variation [30] as well as lack of relevance to the management of the patient [31, 32]. Moreover, DEF was never compared to another imaging modality in order to be recognized as a gold standard for imaging posterior compartment pathologies. This fact raises questions about the accuracy of DEF in detecting pathology, as well as to the determination of the severity of the observed pathology. We suggest that the false positive findings using DTP-US were actually true positive findings, not imagined during DEF. However, this assumption cannot be affirmed due to the fact that DEF was not repeated.
The specificity for the diagnosis of pelvic floor hernias with DTP-US was high in our study. This level of specificity is essential since imaging of the pelvic floor may be indicated in order to correlate symptoms with an anatomical deformation in patients resistant to conservative treatment. Moreover, when surgery becomes a therapeutic option, global evaluation of the pelvic floor is important in order to identify other pelvic floor hernias that might be corrected during the same operation.
The sensitivity for the detection of most pathology was good, although it was only fair for the detection of rectocele. A possible improvement of the sensitivity could be achieved by placing the patient in a supine position on a semi-seated gynecological table.
Our study has limitations. First, we did not correlate the anatomical findings with the clinical status and complains of the examinees. Therefore, the actual clinical value of DEF and DTP-US was not defined. Previous studies demonstrated that structural pelvic floor changes may be demonstrated in asymptomatic patient and that the clinical correlation with symptoms is not obvious [16, 23, 33]. However, this study was designed to compare the accuracy of two imaging examination for the diagnosis of pelvic hernias, a result that was achieved. Another limitation relates to the retrospective design of the study. However, each imaging technique was performed by different operator, which was blinded to the results of the other method.
In conclusion, DTP-US is a relatively low cost, non-irradiating, office procedure. The accuracy of DTP-US in comparison with DEF was found to range from good to excellent, and the specificity of the diagnosis was high for pelvic organ prolapse. The added value of this technique in exploring all the compartments of the pelvic floor as well as the perineal muscles makes DTP-US a preferred procedure for the diagnosis of pelvic floor structural disorders. It can be considered as an ultrasound defecography technique.
References
Nygaard I, Barber MD, Burgio KL et al (2008) Prevalence of symptomatic pelvic floor disorders in US women. JAMA 300:1311–1316
Tally NJ, Weaver AL, Zinsmeister AR et al (1993) Functional constipation and outlet delay: a population based study. Gastroenterology 105:781–190
Felt-Bersma RJ, Luth WJ, Janssen JJ et al (1990) Defecography in patients with anorectal disorders. Which findings are clinically relevant? Dis Colon Rectum 33:277–284
Steensma AB, Oom DM, Burger CW et al (2010) Assessment of posterior compartment prolapse: a comparison of evacuation proctography and 3D transperineal ultrasound. Colorectal Dis 12:533–539
Maglinte DD, Hale DS, Sandrasegaran K (2013) Comparison between dynamic cystocolpoproctography and dynamic pelvic floor MRI: pros and cons: which is the "functional" examination for anorectal and pelvic floor dysfunction? Abdom Imaging 38:952–973
Kelvin FM, Maglinte DD, Benson JT (1994) Evacuation proctography (defecography): an aid to the investigation of pelvic floor disorders. Obstet Gynecol 83:307–314
Maglinte DD, Bartram CI, Hale DA et al (2011) Functional imaging of the pelvic floor. Radiology 258:23–39
Lalwani N, Moshiri M, Lee JH et al (2013) Magnetic resonance imaging of pelvic floor dysfunction. Radiol Clin North Am 51:1127–1139
Beer-Gabel M, Teshler M, Barzilai N, Lurie Y, Malnick S, Bass D, Zbar A (2002) Dynamic transperineal ultrasound in the diagnosis of pelvic floor disorders: pilot study. Dis Colon Rectum 45:239–245
Vitton V, Vignally P, Barthet M et al (2011) Dynamic anal endosonography and MRI defecography in diagnosis of pelvic floor disorders: comparison with conventional defecography. Dis Colon Rectum 54:1398–1404
Martellucci J, Naldini G (2011) Clinical relevance of transperineal ultrasound compared with evacuation proctography for the evaluation of patients with obstructed defaecation. Colorectal Dis 13:1167–1172
Agachan F, Chen T, Pfeifer J et al (1996) A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 39:681–685
Jorge JMN, Wexner SD (1993) Etiology and management of fecal incontinence. Dis Colon Rectum 36:77–97
Drossman DA, Corazziarie E, Delvaux M et al (2006) Rome III. The functional gastrointestinal disorders. Degnon associates, Mclean
DeLancey JOL (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166:1717–1728
Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW (1989) Defecography in normal volunteers: results and implications. Gut 30:1737–1749
Law YM, Fielding JR (2008) MRI of pelvic floor dysfunction: review. AJR 191:S45–S53
Perniola G, Shek C, Chong CCW et al (2008) Defecation proctography and translabial ultrasound in the investigation of defecatory disorders. Ultrasound Obstet Gynecol 31:567–7
Kelvin FM, Hale DS, Maglinte DD et al (1999) Female pelvic organ prolapse: diagnostic contribution of dynamic cystoproctography and comparison with physical examination. AJR Am J Roentgenol 173:31–37
Beer-Gabel M, Assoulin Y, Amitai et al (2008) A comparison of dynamic transperineal ultrasound (DTP-US) with dynamic evacuation proctography (DEP) in the diagnosis of cul de sac hernia (enterocele) in patients with evacuatory dysfunction. Int J Colorectal Dis 23:513–519
Bremmer S, Mellgren A, Holmström B et al (1997) Pelvic anatomy and pathology is influenced by distention of the rectum: defecoperitoneography before and after rectal filling with contrast medium. Dis Colon Rectum 40:1477–1483
Grasso RF, Piciucchi S, Quattrocchi CC et al (2007) Posterior pelvic floor disorders: a prospective comparison using introital ultrasound and colpocystodefecography. Ultrasound Obstet Gynecol 30:86–94
Carter D, Beer-Gabel M (2012) Rectocele—does the size matter? Int J Colorectal Dis 27:975–980
Murthy VK, Orkin BA, Smith LE, Glassman LM (1996) Excellent outcome using selective criteria for rectocele repair. Dis Colon Rectum 39:374–378
Karlbom U, Graf W, Nilsson S et al (1996) Does surgical repair of a rectocele improve rectal emptying? Dis Colon Rectum 39:1296–1302
Kashyap AS, Kohli DR, Raizon A et al (2013) A prospective study evaluating emotional disturbance in subjects undergoing defecating proctography. World J Gastroenterol 19:3990–3995
Halligan S, Spence-Jones C, Kamm MA et al (1996) Dynamic cystoproctography and physiological testing in women with urinary stress incontinence and urogenital prolapse. Clin Radiol 51:785–790
Healy JC, Halligan S, Reznek RH et al (1997) Patterns of prolapse in women with symptoms of pelvic floor weakness: assessment with MR imaging. Radiology 203:77–81
Goei R, Kemerink G (1990) Radiation dose in defecography. Radiology 176:137–139
Müller-Lissner SA, Bartolo DC et al (1998) Interobserver agreement in defecography—an international study. Z Gastroenterol 36:273–279
Ott DJ, Donati DL, Kerr RM et al (1994) Defecography: results in 55 patients and impact on clinical management. Abdom Imaging 19:349–354
Hiltunen KM, Kolehmainen H, Matikainen M (1994) Does defecography help in diagnosis and clinical decision-making in defecation disorders? Abdom Imaging 19:355
Dvorkin LS, Gladman MA, Epstein J, Scott SM, Williams NS, Lunniss PJ (2005) Rectal intussusception in symptomatic patients is different from that in asymptomatic volunteers. Br J Surg 92:866–872
Author information
Authors and Affiliations
Corresponding author
Additional information
Beer- Gabel holds a MD, FEBGH, Chaim Sheba Medical Center.
Carter holds a MD. FEBGH, Chaim Sheba Medical Center.
Rights and permissions
About this article
Cite this article
Beer-Gabel, M., Carter, D. Comparison of dynamic transperineal ultrasound and defecography for the evaluation of pelvic floor disorders. Int J Colorectal Dis 30, 835–841 (2015). https://doi.org/10.1007/s00384-015-2195-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2195-9