Abstract
Purpose
The risk factors for acquiring an infection with multidrug-resistant (MDR) bacteria in patients with anastomotic leakage after colorectal cancer surgery are poorly understood. We evaluated the risk factors associated with the initial acquisition of MDR pathogens in patients with anastomotic leakage after colorectal cancer surgery.
Methods
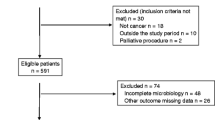
This study was a retrospective review of prospectively collected data at a university affiliated-tertiary referral hospital in South Korea. From January 2009 to April 2013, a total of 6767 consecutive patients with colorectal cancer who underwent surgery were registered. Of these patients, 190 (2.8 %) were diagnosed with anastomotic leakage. Finally, 143 (2.1 %) patients with culture test results were included in this study.
Results
Of the 143 enrolled patients, 46 (32.2 %) were classified in the MDR group. The use of antibiotics for more than 5 days before diagnosis of anastomosis site leakage (p = 0.016) and diabetes mellitus (p = 0.028) was identified as independent risk factors for MDR acquisition by multivariate analysis. The rate of adequate initial empirical antibiotic therapy in the MDR group was lower than in the non-MDR group (35 vs. 75 %, p < 0.001). Furthermore, the duration of antibiotic administration after the leak was longer in the MDR group (p = 0.013). Patients in the MDR group also had a longer hospital stay (p = 0.012).
Conclusions
The length of antibiotic administration before the diagnosis of anastomotic leakage and diabetes mellitus were risk factors associated with the acquisition of MDR bacteria in patients with anastomotic leakage after colorectal cancer surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative peritonitis, a serious complication following colorectal surgery, is associated with a high rate of septic shock (41 %) and mortality (19–40 %) [1, 2]. Furthermore, the treatment of postoperative peritonitis necessitates the use of significant hospital resources and is therefore costly [3]. In particular, anastomotic leakage accounts for 34 % of all cases of postoperative peritonitis [4], while the mortality rate associated with leakage after colorectal surgery is 8 % [5]. In spite of new operating techniques and materials, anastomotic leakage after colorectal surgery remains prevalent [6, 7]. Two treatment modalities exist for anastomotic leakage: operative or nonoperative management. In both cases, the timely administration of suitable antibiotics is essential [8]. In recent years, the incidence of antimicrobial-resistant pathogens has increased rapidly, which has become a significant challenge for clinicians worldwide [9]. Inappropriate antimicrobial therapy for multidrug-resistant (MDR) infection is associated with significantly poorer outcomes [10–12]. The identification of the clinical characteristics that define patients at high risk for the acquisition of MDR bacteria could aid clinicians in their choice of antibiotics for patients with anastomotic leakage after colorectal cancer surgery. However, the risk factors, clinical features, and outcomes of patients who have MDR bacterial acquisition and anastomotic leakage after colorectal cancer surgery are not well understood.
In this study, we identified independent risk factors for the acquisition of MDR pathogens at the time of diagnosis of anastomotic leakage after colorectal cancer surgery. We also evaluated risk factors for MDR bacterial acquisition in the early phase of leakage to improve early antibiotic therapy after diagnosis of leakage. We compared the outcomes of patients with MDR pathogens to patients without MDR pathogens.
Materials and methods
Study design and study population
A retrospective cohort study was conducted. The local Institutional Review Board approved the study and waived the requirement for informed consent (IRB No. 2013-10-067-001).
From January 2009 to April 2013, a total of 6767 patients with colorectal cancer underwent surgery in the Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine. Of these patients, 2302 had low anterior resections. There were 1870 anterior resections, 1436 right colectomies, 283 left colectomies, 73 total colectomies, 42 intersphincteric resections, 38 transverse colectomies, and 723 nonanastomotic operations. Anastomotic leakage occurred in 190 patients (2.8 %): 138 (6.0 %) low anterior resection patients, 17 (0.9 %) anterior resection patients, 24 (1.7 %) right hemicolectomy patients, 6 (2.1 %) left hemicolectomy patients, 4 (5.5 %) total colectomy patients, and 1 (2.6 %) transverse colectomy patient. Of the 190 patients screened, 47 patients did not have blood or intra-abdominal fluid culture tests on the first day after diagnosis of anastomotic leakage and were thus excluded; therefore, a total of 143 patients were enrolled in this study.
The routine procedure for colorectal cancer surgery at our institution was as follows: Antibiotic prophylaxis using second-generation cephalosporin was administered, ideally within the hour prior to incision; the duration of antibiotic administration did not exceed 24 h. The bowel was prepared for surgery with an oral citric acid (19.5 g)/magnesium carbonate (10.75 g) solution and oral bisacodyl (10 mg) or 2000 ml of balanced lavage solution (containing 210 g of polyethylene glycol) in cases of low anterior resection without fecal diversion. Preoperative nonabsorbable oral antibiotics were not used for bowel preparation. From 2009 to 2011, feeding was delayed until the return of bowel function (defined as the presence of bowel sounds, passage of flatus, or stool). Starting in 2012, our institution has employed an early feeding strategy in which patients started a clear liquid diet on the first postoperative day and advanced to a regular diet after liquids were well tolerated.
Microbiological data
All culture tests were performed on the first day after diagnosis of anastomotic leakage; bacteriological profiles were based on these tests. Bacterial culture tests performed before anastomotic leakage or in the 2 days following anastomotic leakage were not included in this study. In cases of reoperation, abdominal fluid was taken for culture from the operative field, or in the immediate postoperative period, through closed suction drainage. If the patients had undergone percutaneous catheter drainage instead of reoperation, their drainage fluid was used. Blood and abdominal fluid cultures were not routinely ordered, and the need for these cultures was determined by the doctor on duty based on the condition of the patient in question.
Definitions
Patients with anastomotic leakage were defined as patients with confirmed anastomotic leakage at reoperation or patients for whom disruption of the anastomosis had been suspected and then confirmed by a colorectal surgeon based on imaging studies and clinical examination. Simple abscess or intra-abdominal fluid collection without any symptoms or signs of leakage was not considered to be anastomotic leakage.
The definition of MDR gram-positive and gram-negative organisms (i.e., methicillin-resistant Staphylococcus aureus [MRSA], extended-spectrum cephalosporin-resistant Klebsiella pneumoniae, Escherichia coli, and carbapenem-resistant Pseudomonas aeruginosa) was first described by Magiorakos et al. [13] and included resistance to one key antimicrobial agent. Briefly, it was defined as acquired nonsusceptibility to at least one agent in three or more antimicrobial categories.
Total antibiotic administration period before leakage was defined as the number of days that antibiotics were administered prior to diagnosis of leakage (including pre- and postoperative periods before diagnosis of leakage but during the same hospitalization).
Data collection and statistical analysis
All clinical data from patients with colorectal cancer who underwent colorectal cancer surgery were prospectively included in the colorectal cancer database of our hospital, and the medical records of all patients were retrospectively reviewed. Basic demographic parameters including age, sex, comorbidities, neo-adjuvant chemotherapy, operating report, microbial isolates and susceptibilities, mortality, peak temperature and systolic blood pressure on the day of diagnosis of anastomotic leakage, time from operation to leakage, duration of antibiotics administration, and duration of hospital stay were recorded. To assess potential relationships between patient outcomes and infections with MDR pathogens, the following parameters were included: vital signs 1 day before and 1 day after leakage, bacteriological profiles, treatment methods, length of hospital stay, antibiotics, and in-hospital mortality.
Continuous variables were compared using the Mann–Whitney U test and Student’s t test. Qualitative variables were compared using the chi-square (χ 2) test. Backward stepwise logistic regression analysis was used to control for the effects of confounding variables while identifying independent risk factors for acquisition of MDR pathogens. All risk factors with an association of p < 0.2 at the bivariate level were included in the multivariate logistic model to predict the acquisition of MDR pathogens. Continuous variables were recoded using receiver operating characteristic curves to define the appropriate cutoff points (represented by the values with the best sensitivity and specificity). The odds ratios (ORs) and their associated 95 % confidence intervals (CIs) were also calculated. For all analyses, a p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc., Chicago, IL).
Results
Study population
Of the 143 patients, more were males (81.8 %) than females (18.2 %). The mean patient age was 58.3 ± 10.4 years. The median hospital stay was 24 days [interquartile range (IQR), 17–34 days]. The median duration of antibiotic administration after leakage was 11 days (IQR, 7–21 days), and adequate initial empirical antibiotic therapy was given to 89 patients (62.2 %). Anastomotic leakage was most common after rectal surgery (n = 121, 84.6 %), followed by right colon surgery (n = 17, 11.9 %) and left colon surgery (n = 5, 3.5 %).
Clinical characteristics of the MDR and non-MDR groups
Of the 143 patients, 115 patients (80.4 %) were reported as having “growth,” with the remaining 28 (19.6 %) having “no growth.” A total of 232 microorganisms were cultured from either patient blood or abdominal fluid (Table 1) and 46 patients (32.2 %) were classified in the MDR group. The MDR bacteria isolated were the following: MDR Enterococcus spp. (n = 31), MDR Enterobacter spp. (n = 9), MDR P. aeruginosa (n = 4), ESBL E. coli (n = 4), Acinetobacter spp. (n = 3), and MRSA (n = 1). Patient baseline and demographic characteristics according to the group (MDR vs. non-MDR) are shown in Table 2. Univariate analysis revealed that a history of ICU admission after the first operation (p = 0.048) and diabetes mellitus (p = 0.032) were all factors associated with the acquisition of MDR pathogens in anastomotic leakage. However, age, sex, hospitalization within the 3-month period prior to admission, total length of antibiotic administration before diagnosis of anastomotic leakage, neo-adjuvant chemotherapy, protective ileostomy, bowel preparation status, and the presence of intestinal obstruction were not significant factors. In 134 patients (94 %), second-generation cephalosporin was administered as a preoperative antibiotic, but six patients (4 %) with suspicion of preoperative peritonitis were prescribed third-generation cephalosporin. Three patients with cephalosporin allergies (2 %) received quinolone without β-lactams. No relationship was observed between the preoperative antibiotics administered and the acquisition of MDR pathogens. In addition, the duration from initial admission to diagnosis of leakage, the duration from the first operation to leakage, and the site of anastomotic leakage were not associated with the acquisition of MDR pathogens. The total length of antibiotic administration before the diagnosis of anastomotic leakage was transformed into a binary variable using a cutoff point of 5 days, as determined by the receiver operating characteristic curve, to give the best sensitivity (50.0 %) and specificity (70.1 %). In a multiple logistic regression analysis, after adjusting for potential confounding factors, diabetes mellitus (adjusted OR, 2.96; 95 % CI, 1.13–7.81; p = 0.028) and the total length of antibiotic administration for more than 5 days before diagnosis of anastomosis site leakage (adjusted OR, 2.48; 95 % CI, 1.18–5.20; p = 0.016) significantly associated with MDR pathogen acquisition. The Hosmer-Lemeshow goodness-of-fit test showed good agreement between the observed and predicted values of the model (p = 0.768).
In the subgroup analysis, 116 patients performed abdominal fluid culture irrespective of blood culture, history of ICU admission after the first operation (p = 0.040), diabetes mellitus (p = 0.029), and minimal invasive surgery (p = 0.029), which were all factors associated with the acquisition of MDR pathogens in anastomotic leakage. In the multiple logistic backward regression analysis, diabetes mellitus (adjusted OR 3.18; 95 % CI, 1.01–9.98; p = 0.048), the total length of antibiotics administration per day (adjusted OR 1.20; 95 % CI, 1.02–1.41; p = 0.032), and hospitalization within the 3-month period prior to admission (adjusted OR 6.44; 95 % 1.15–35.01; p = 0.034) significantly associated with MDR pathogen acquisition (see details in the online supplement).
Clinical features, antibiotics, and outcomes
Patient clinical features and outcomes are presented in Table 3. Temperature, treatment method, and in-hospital mortality were similar between the two groups. Out of the 111 patients for whom blood cultures were available, bacteremia was more frequent in the patients with MDR pathogens compared with the patients with non-MDR pathogens; however, this trend was not statistically significant (22.2 % for MDR vs. 13.3 % for non-MDR; p = 0.234). Hypotension (systolic blood pressure <90 mmHg) 1 day before and 1 day after leakage was more frequent in patients with MDR pathogens (37 vs. 13 %; p = 0.001), and the length of hospital stay in the MDR group was longer than in the non-MDR group (30 vs. 24 days, p = 0.012). In the MDR group, initial empirical antibiotics were adequate for 35 % (16/46) of all patients. On the other hand, in the non-MDR group, initial antibiotics were adequate for 76 % (73/97) of all patients. The average duration of antibiotic administration after leakage was also longer in the MDR group (14.5 vs. 10 days, p = 0.013).
We analyzed the initial empirical antibiotics prescribed at the time of leakage. Piperacillin/tazobactam (pip/taz; n = 56) and cephalosporin (second-generation cephalosporin = 35, third-generation cephalosporin = 24) were the main antimicrobial agents prescribed. In the MDR group, cephalosporin and pip/taz achieved poor adequacy rates, but in the non-MDR group, pip/taz achieved an initial empirical therapy adequacy rate above 90 %. Cephalosporin-based therapy yielded poor results, even in the non-MDR group.
Discussion
Previous studies examining postoperative peritonitis have tended to include a broad variety of pathological conditions, such as community or nosocomial settings, sites of primary surgical procedure, mechanisms of peritonitis, and underlying cancer [4, 14, 15]. However, to our knowledge, this is the first study to focus on patients with anastomotic leakage after colorectal cancer surgery.
In this study, Enterococcus spp. were the most dominant bacteria, followed by Enterobacter, P. aeruginosa, Bacteroides fragilis, E. coli, K. pneumonia, etc. (Table 1). The bacteriological profiles found in our study population were similar to those previously described in postoperative peritonitis [4, 15]. Our study revealed a 32.2 % incidence of MDR pathogens in patients with anastomotic leakage after colorectal cancer surgery. The bacteriological profiles were similar between the MDR and non-MDR groups (Table 1). The incidence of MDR pathogen acquisition was similar to figures reported in other postoperative peritonitis studies [10, 15].
Peritonitis is typically classified into three categories: community-acquired peritonitis, nosocomial postoperative peritonitis, and nosocomial nonpostoperative peritonitis (occurring without surgical intervention) [14]. Each category generally entails a different treatment strategy. In community-acquired peritonitis, antimicrobial selection focuses on Enterobacteriaceae, especially E. coli and anaerobes, while the most suitable course of therapy against Enterococci remains controversial [11, 16–18]. Routine coverage against Enterococcus is not necessary for patients with community-acquired intra-abdominal infections [19–21]. However, antimicrobial therapy for Enterococci should be given when Enterococci are recovered from patients with nosocomial infections [11]. In the nosocomial setting, more resistant flora may be considered, including Enterococcus, P. aeruginosa, Enterobacter spp., MRSA, and Candida species. In this setting, it has also been shown that inadequate antimicrobial therapy prolongs hospitalization and is associated with poor outcomes [10–12, 22]. Thus, expanded spectrum multidrug regimens, which include either an aminoglycoside or a quinolone or a carbapenem and vancomycin, are recommended for these nosocomial infections [11].
The extent of leakage determined whether operative or nonoperative treatment would be used. Usually, patients with a simple abscess or intra-abdominal fluid collection without any symptoms or signs of leakage were treated in a nonoperative manner. To maximize the homogeneity of the study population, patients with a simple abscess or intra-abdominal fluid collection without any symptoms or signs of leakage were excluded. Our study focused on the acquisition of MDR bacteria at the time of diagnosis of leakage, irrespective of the extent of leakage or severity of peritonitis, and patients who underwent both operative and nonoperative interventions were analyzed to evaluate risk factors for acquisition of MDR pathogens.
Expanded spectrum multidrug regimens are important for patients with nosocomial postoperative peritonitis; however, it is perhaps more important to identify the relevant risk factors for acquiring an MDR pathogen among this group of patients. A prolonged preoperative length of stay and a prolonged (more than 2 days) period of preoperative antimicrobial therapy have both been shown to be related to resistance to empirical antimicrobial regimens; thus, such patients should be treated for nosocomial infection [11]. According to Seguin et al. [15], a prolonged preoperative hospital stay (>5 days) was associated with the acquisition of MDR pathogens. However, in the present study, the median duration from patient admission to leakage was not different between the MDR and non-MDR groups [median (IQR); 7 (6–10) for MDR group vs. 7 (5–11) for non-MDR group, p = 0.760]. We also found that diabetes mellitus and the administration of antibiotics more than 5 days before the diagnosis of anastomotic site leakage were both strong independent risk factors for the acquisition of an MDR pathogen. Hospitalization within 3 months before admission also tended to be associated with the acquisition of an MDR pathogen. Thus, appropriate therapeutic approaches can be proposed based on the analysis of risk factors for the acquisition of MDR bacteria. For instance, complex multidrug regimens may be suitable empirical antimicrobial agents after leakage in patients with diabetes mellitus or in patients who underwent administration of antibiotics ≥5 days before the diagnosis of anastomotic site leakage. This proposal is suited for patients in a nosocomial setting, and the most suitable regimen may differ according to local epidemiology. Recent guidelines have emphasized the importance of early extended-spectrum empirical antibiotics, followed by rapid de-escalation after microbiologic identification of pathogens and susceptibility tests [8, 11, 23].
In this study, we observed that patients in the MDR group received inadequate empirical antimicrobial therapy more frequently than patients in the non-MDR group, and also that the average duration of antibiotic administration was longer in the MDR group. In addition, the median duration of patient hospitalization was longer in the MDR group. Hypotension was more likely to occur in patients who had recovered from infections with MDR pathogens. However, mortality rates were not significantly different between patients with and without MDR pathogens. Inadequate antibiotic selection has been shown in several studies to prolong hospital stay and has also been associated with a poor clinical outcome (prolonged hospital stay, treatment failure, and a higher mortality rate) [8, 22]. However, other studies of nosocomial peritoneal infections did not observe any relationships between inadequate antibiotic selection and poor clinical outcomes [4, 15, 24]. Although this perceived lack of effect on clinical outcomes may actually be due to a lack of power due to small sample sizes, the effect of inappropriate empirical antimicrobial selection on the outcomes of patients with postoperative peritonitis still remains controversial [1, 10]. In fact, a more critical aspect of managing postoperative peritonitis is surgical intervention, which can both control the source of infection and also decrease the bacterial load [4]. It is also well known that in cases of septic shock, each hour of delay in achieving administration of effective antibiotics is accompanied by a measurable increase in mortality [8]. Also, according to a surviving sepsis campaign, patients with severe sepsis or septic shock warrant broad-spectrum therapy until the causative organism and its antibiotic susceptibilities are defined [25]. For these reasons, and despite the uncertain link between outcome and inadequate empirical antibiotic selection, prompt initial antibiotic administration appears to be crucial for survival, at least for patients with severe sepsis or septic shock.
Our study does have several limitations. First, this study entailed a retrospective analysis of a prospectively collected database at a single center. Thus, hidden bias may have led to under- or overestimation of the true relationship between the risk factors and the acquisition of MDR pathogens. However, we attempted to address this limitation by performing multivariate logistic analyses to control for confounding factors. Second, blood or intra-abdominal culture tests were not conducted in 47 of the 190 patients with anastomotic leakage. Thus, the absence of culture tests for these 47 patients may have influenced the conclusions of this study. Third, detailed information for calculating severity scores was not available in our database. Therefore, the severity scores, which were not adjusted in our analysis, may have obscured the true result. However, all patients in this study had colorectal cancer, and in all cases, surgery was elective. Finally, up to the present, there was no universal definition of anastomotic leakage. The definition of anastomotic leakage used in this study was not perfect; however, the definition was deemed the most simple and least prone to observer bias [7]. Nevertheless, it is important to note that the patient population was focused on anastomotic leakage after colorectal cancer surgery, and also that this study is the largest study published to date.
Anastomotic leakage is one of the most serious complications of colorectal surgery. Most previous reports have focused on leakage rates, risk factors for leakage, mortality, and oncological and bowel function outcomes. However, the epidemiology and clinical features of MDR bacteria in patients with anastomotic leakage after colorectal cancer surgery have not yet been identified. The identification of the clinical characteristics that define patients at high risk for the acquisition of MDR bacteria could aid clinicians in the appropriate management of these life-threatening conditions.
Conclusions
In patients with anastomotic leakage after colorectal cancer surgery, the incidence of MDR pathogen acquisition was 32.2 %. The acquisition of MDR pathogens was significantly associated with diabetes mellitus and the use of antibiotics for more than 5 days before the diagnosis of anastomotic leakage. In the context of these risk factors, complex multidrug regimens should be considered when selecting the appropriate empirical antibiotic therapy prior to a confirmed bacteriological profile in patients diagnosed with leakage after colorectal cancer surgery.
References
Roehrborn A, Thomas L, Potreck O, Ebener C, Ohmann C, Goretzki PE, Roher HD (2001) The microbiology of postoperative peritonitis. Clin Infect Dis 33:1513–1519
Riche FC, Dray X, Laisne MJ, Mateo J, Raskine L, Sanson-Le Pors MJ, Payen D, Valleur P, Cholley BP (2009) Factors associated with septic shock and mortality in generalized peritonitis: comparison between community-acquired and postoperative peritonitis. Crit Care 13:R99
Frye J, Bokey EL, Chapuis PH, Sinclair G, Dent OF (2009) Anastomotic leakage after resection of colorectal cancer generates prodigious use of hospital resources. Color Dis 11:917–920
Augustin P, Kermarrec N, Muller-Serieys C, Lasocki S, Chosidow D, Marmuse JP, Valin N, Desmonts JM, Montravers P (2010) Risk factors for multidrug resistant bacteria and optimization of empirical antibiotic therapy in postoperative peritonitis. Crit Care 14:R20
Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R (2004) Risk factors for anastomotic leakage after anterior resection of the rectum. Color Dis 6:462–469
Eckmann C, Kujath P, Schiedeck TH, Shekarriz H, Bruch HP (2004) Anastomotic leakage following low anterior resection: results of a standardized diagnostic and therapeutic approach. Int J Color Dis 19:128–133
Caulfield H, Hyman NH (2013) Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg 148:177–182
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Gold HS, Moellering RC Jr (1996) Antimicrobial-drug resistance. N Engl J Med 335:1445–1453
Montravers P, Gauzit R, Muller C, Marmuse JP, Fichelle A, Desmonts JM (1996) Emergence of antibiotic-resistant bacteria in cases of peritonitis after intraabdominal surgery affects the efficacy of empirical antimicrobial therapy. Clin Infect Dis 23:486–494
Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, Buchman T, Dellinger EP, Jernigan J, Gorbach S, Chow AW, Bartlett J (2003) Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis 37:997–1005
Christou NV, Turgeon P, Wassef R, Rotstein O, Bohnen J, Potvin M (1996) Management of intra-abdominal infections. The case for intraoperative cultures and comprehensive broad-spectrum antibiotic coverage. The Canadian Intra-abdominal Infection Study Group. Arch Surg 131:1193–1201
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Seguin P, Laviolle B, Chanavaz C, Donnio PY, Gautier-Lerestif AL, Campion JP, Malledant Y (2006) Factors associated with multidrug-resistant bacteria in secondary peritonitis: impact on antibiotic therapy. Clin Microbiol Infect 12:980–985
Seguin P, Fedun Y, Laviolle B, Nesseler N, Donnio PY, Malledant Y (2010) Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J Antimicrob Chemother 65:342–346
Burnett RJ, Haverstock DC, Dellinger EP, Reinhart HH, Bohnen JM, Rotstein OD, Vogel SB, Solomkin JS (1995) Definition of the role of Enterococcus in intraabdominal infection: analysis of a prospective randomized trial. Surgery 118:716–721, discussion 721–713
Montravers P, Andremont A, Massias L, Carbon C (1994) Investigation of the potential role of Enterococcus faecalis in the pathophysiology of experimental peritonitis. J Infect Dis 169:821–830
Sitges-Serra A, Lopez MJ, Girvent M, Almirall S, Sancho JJ (2002) Postoperative enterococcal infection after treatment of complicated intra-abdominal sepsis. Br J Surg 89:361–367
Walker AP, Nichols RL, Wilson RF, Bivens BA, Trunkey DD, Edmiston CE Jr, Smith JW, Condon RE (1993) Efficacy of a beta-lactamase inhibitor combination for serious intraabdominal infections. Ann Surg 217:115–121
Cohn SM, Lipsett PA, Buchman TG, Cheadle WG, Milsom JW, O'Marro S, Yellin AE, Jungerwirth S, Rochefort EV, Haverstock DC, Kowalsky SF (2000) Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann Surg 232:254–262
Ohlin B, Cederberg A, Forssell H, Solhaug JH, Tveit E (1999) Piperacillin/tazobactam compared with cefuroxime/ metronidazole in the treatment of intra-abdominal infections. Eur J Surg 165:875–884
Krobot K, Yin D, Zhang Q, Sen S, Altendorf-Hofmann A, Scheele J, Sendt W (2004) Effect of inappropriate initial empiric antibiotic therapy on outcome of patients with community-acquired intra-abdominal infections requiring surgery. Eur J Clin Microbiol Infect Dis 23:682–687
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
Montravers P, Lepape A, Dubreuil L, Gauzit R, Pean Y, Benchimol D, Dupont H (2009) Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother 63:785–794
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637
Funding/support
None.
Financial disclosure
None.
Conflict of interest
The authors declared no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, DS., Ryu, JA., Chung, C.R. et al. Risk factors for acquisition of multidrug-resistant bacteria in patients with anastomotic leakage after colorectal cancer surgery. Int J Colorectal Dis 30, 497–504 (2015). https://doi.org/10.1007/s00384-015-2161-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-015-2161-6