Abstract
Background/purpose
Living donor liver transplantation (LDLT) is vital for pediatric end-stage liver disease due to organ shortages. The graft-to-recipient weight ratio (GRWR) preoperatively measured predicts the outcomes of LDLT. We typically target between 0.8 and 3.0–4.0%, but the ideal GRWR remains controversial. We compared the outcomes of LDLT according to the GRWR to examine whether the criteria could be expanded while ensuring safety.
Methods
We retrospectively reviewed 99 patients who underwent LDLT in our department by dividing them into three groups according to their GRWR: Group S, with GRWR values lower than the normal range (GRWR < 0.8%); Group M, with GRWR values in the normal range (GRWR ≥ 0.8 to < 3.5%); and Group L, with GRWR values above the normal range (GRWR ≥ 3.5%).
Results
In Groups S and L, 46.2 and 44.4% of patients underwent splenectomy and delayed abdominal wall closure, respectively. After these intraoperative adjustments, there were no significant differences between the groups in 5-year patient survival, 5-year graft survival, or the occurrence of post-transplantation thrombosis.
Conclusion
When the GRWR is beyond the normal threshold, the risk of complications associated with graft size might be reduced by adjustments to provide appropriate portal blood flow and by delayed abdominal wall closure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
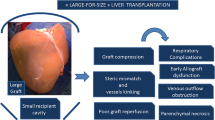
Liver transplantation (LT) is widely accepted as a curative treatment for end-stage liver disease in children. Currently, living donor liver transplantation (LDLT) is widely performed, especially in Japan, due to the severe shortage of cadaveric organs. In pediatric LDLT using adult donors, graft–recipient size mismatch remains a surgical challenge that can lead to graft failure and patient death if not properly addressed [1]. In infant recipients, left lateral segment (LLS) grafts from adult donors, reduced LLS grafts, or mono-segmental grafts have been used to adjust for size [2, 3]; however, they may still be too large for some infants.
In contrast, the grafts may be too small to meet the metabolic demands of the recipient in recipients who have reached puberty or adulthood. To maximize donor safety, the graft size that can be procured must be limited. Thus, the main limitation of LDLT is whether this size is appropriate for recipients who have reached adolescence or adulthood [4]. Undersized grafts can cause a condition, called small-for-size syndrome, and are mainly characterized by massive ascites, prolonged cholestasis, and coagulopathy after transplantation, sometimes leading to graft dysfunction and hepatic encephalopathy [5, 6].
The graft-to-recipient weight ratio (GRWR) has been used as a predictor of graft size mismatch and has been reported to be associated with post-transplant outcomes [7]. Previous studies have indicated that the ideal GRWR range to avoid major complications related to size mismatch should be between 0.8 and 3.0–4.0% [8,9,10,11]. However, the impact of graft–recipient size mismatch on LDLT outcomes is unclear due to some reports deviating from this range [12,13,14,15]. In addition, expanding the GRWR criteria while ensuring safety could be a solution to the donor shortage. Therefore, the purpose of this study was to review our single-center experience, retrospectively examine the post-transplant outcomes of patients with graft size mismatch in LDLT, and evaluate the safety of the procedure.
Materials and methods
From January 1996 to December 2022, 137 patients underwent LDLT at Kyushu University Hospital, with the approval of the Kyushu University Ethics and Indications Committee. After excluding 38 cases with missing data, a total of 99 cases were included in the present study. First, we divided the 99 patients into three groups according to their GRWR: Group S, with GRWR values lower than the normal range (GRWR < 0.8%; n = 13); Group M, with GRWR values within the normal range (GRWR ≥ 0.8% to < 3.5%; n = 77); and Group L, with GRWR values above the normal range (GRWR ≥ 3.5%). We then retrospectively reviewed their demographic data, disease-related data, intraoperative data, biochemical profiles, and postoperative complications from medical records.
Donor data
The donors were willing to volunteer as living donors, spouses or within third-degree consanguinity of the recipient, and 20–60 years of age. We performed computed tomography (CT) of the donors for a volumetric analysis and delineation of the vascular anatomy. Using the 3D image analysis application SYNAPSE VINCENT®, we estimated the volume of the graft and the donor’s remnant liver volume. When the estimated remnant liver volume of the donor was < 35% of the total liver volume, the donor was rejected. In this study, we retrospectively collected donor sex and age data from medical records.
Recipient data
We calculated the standard liver volumes of the recipients using Urata’s formula as follows [16]: liver volume (ml) = body surface area (BSA; m2) × 706.2 + 2.4. We determined the graft type for each recipient based on the graft volume/standard liver volume ratio (GV/SLV) and calculated the GRWR from the estimated graft volume, standard liver volume, and recipient weight. The approximate target for the GV/SLV ratio was ≥ 35%, while the approximate target for the GRWR was ≥ 0.8 to 3.5%. In some cases, multiple types of grafts met the above criteria; in others, regardless of the type of graft selected, the above criteria could not be met, and the appropriate type was selected based on the medical condition. We retrospectively collected recipient data such as the age at LDLT, sex, weight at LDLT, indication for LDLT, and ABO match from the medical records. In addition, we calculated the MELD score for recipients older than 12 years and the PELD score for recipients younger than 12 years to compare the liver function before transplantation. We used the online MELD or PELD calculator based on previously described formulas [17, 18]: MELD = 3.78 × log[TB (mg/dL)] + 11.2 × log[INR] + 9.57 × log[serum creatinine (mg/dL)] + 6.43, PELD = 0.436 (age (< 1 year)) − 0.687 × log (serum albumin (g/dL)) + 0.480 × log (TB (mg/dL)) + 1.857 × log (INR) + 0.667 (growth retardation (< − 2 SD)).
Intraoperative data
Based on the preoperative decisions, we procured grafts from donors. After anastomosing the graft with the recipients’ vessels, we measured the portal and hepatic arterial blood flow velocities. Patients with preoperative portal hypertension and splenomegaly had a high portal blood flow velocity. In such cases, we ligated the collateral blood vessels or performed splenectomy according to the surgeon's preference to adjust the portal blood flow rate. The abdominal wall closure method was selected based on the tension created by suturing the skin and muscles at the end of the transplant. In cases where there is concern that a closed wound will reduce graft blood flow or cause abdominal compartment syndrome, we cut a polytetrafluoroethylene sheet (Gore-Tex sheet®) to size and sutured it circumferentially to the fascia.
We collected clinical data, including the operation time, anhepatic period, cold and warm ischemic time, post-anastomotic portal vein flow (PVF), and post-anastomotic hepatic artery flow (HAF). To correct for differences in body size in each group, we calculated portal blood flow velocity per graft weight (PVF/GV) and hepatic artery blood flow velocity per graft weight (HAF/GV).
Postoperative data
We compared the patient and graft survival rates, as well as the ratio of portal vein thrombosis (PVT), hepatic artery thrombosis (HAT), hepatic vein thrombosis (HVT), complications related to the bile duct, and other complications. We also compared other clinical data, such as the postoperative biochemical and coagulation profile on postoperative days (PODs) 1, 3, 7, 14, 21, and 28, the volume of ascites on POD7, and complications.
Statistical analysis
The Chi-square test and Student’s t test were used for continuous data to assess the significance of differences between each group. Values are expressed as the mean ± standard deviation. Categorical variables are expressed as numbers and percentages. Fisher’s exact test was used to determine the significance of differences. Cumulative analyses were performed using the Kaplan–Meier method with the Wilcoxon test. We compared the clinical data of Groups S and L with the data of Groups M. P values of < 0.05 were considered to indicate statistical significance. P values between Group S and Group M are denoted as pSM, while those between Group M and Group L are denoted as pML. All statistical analyses were performed using JMP (version 16.0; SAS Institute, Cary, NC, USA).
This study was conducted in accordance with the Ethical Guidelines for Clinical Research published on July 30 (revised 2008) by the Ministry of Health, Labor, and Welfare of Japan and complied with the 1964 Helsinki Declaration (revised 2008). Our university’s institutional review board approved this study (No. 23348-00).
Results
Patient characteristics
Table 1 presents the patient demographic data. The mean age of recipients at LDLT was significantly older in Group S (17.1 ± 4.3 years) and younger in Group L (0.6 ± 0.1 years) than in Group M (3.9 ± 6.0 years) (pSM < 0.01, pML = 0.09). The mean age of donors was also significantly older in Group S (42.2 ± 9.7 years) and younger in Group L (29.8 ± 5.6 years) than in Group M (35.3 ± 7.9 years) (pSM < 0.01, pML = 0.05). There was no significant difference between the groups in terms of the indications for LDLT or ABO blood type compatibility. The pretransplant liver function, as estimated by the MELD score, in Groups S and M did not differ to a statistically significant extent (S vs. M = 15.2 ± 8.1 vs. 10.7 ± 1.5; pSM = 0.2). However, there was a significant difference in the PELD scores of Groups M and L (M vs. L = 11.5 ± 12.1 vs. 21.3 ± 8.7; pML = 0.02).
Intraoperative data
The intraoperative data are summarized in Table 2. More cases in Group S used extended left lobe grafts (n = 8, 61.5%), whereas more cases in Group L used left lateral segment grafts (n = 8, 88.9%). There was a significant difference in the distribution of graft types between Groups S and M (pSM < 0.001; pML = 0.53). There was also a significant difference between the groups in GV/SLV (S vs. M vs. L = 38.6 ± 9.0% vs. 67.8 ± 18.2% vs. 102.5 ± 2.6%; pSM < 0.001, pML < 0.001) and GRWR (S vs. M vs. L = 0.69 ± 0.1% vs. 2.3 ± 0.8% vs. 3.9 ± 0.3%; pSM < 0.001, pML < 0.001). The operating time in Group S was significantly longer than that in Groups M and L (S vs. M vs. L = 908.3 ± 243.8 min vs. 720.2 ± 149.0 min vs. 749.7 ± 169.5 min; pSM < 0.001, pML = 0.61). We could not find any difference in the anhepatic period, cold ischemic time, or warm ischemic time between the groups.
The PVF was significantly higher in Group S and lower in Group L than in Group M (S vs. M vs. L = 1061.3 ± 287.9 ml/min vs. 478.0 ± 426.6 ml/min vs. 211.9 ± 147.1 ml/min; pSM < 0.001, pML = 0.06). On the other hand, when the PVF/GV values were compared, we found a significant difference between Groups M and L, but not between Groups S and M (S vs. M vs. L = 273.2 ± 86.8 ml/min/100 g vs. 478.0 ± 426.6 ml/min/100 g vs. 93.3 ± 58.7 ml/min/100 g; pSM = 0.06, pML = 0.01). No significant difference was seen in HAF/GV (S vs. M vs. L = 273.2 ± 86.8 ml/min/100 g vs. 478.0 ± 426.6 ml/min/100 g vs. 93.3 ± 58.7 ml/min/100 g; pSM = 0.06, pML = 0.01). Splenectomy was performed to regulate portal blood flow in 6 (46.2%) of the 13 patients in Group S, which was a significantly higher rate than that in Group M. In addition, 44.4% (four cases) of the patients in Group L underwent delayed abdominal wall closure, which was a significantly higher rate than that in Group M.
Postoperative data
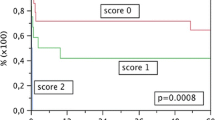
There were no cases of early death or re-transplantation in Groups S or L, with the exception of one patient in Group L who died of graft failure at 95 days after transplantation. Figure 1 shows the Kaplan–Meier survival curves. The 5-year survival rates were 100% in Group S and 88.9% in Group L; these values were not significantly different from the survival rate of Group M (90.8%). The graft survival rates of the groups did not differ to a statistically significant extent.
The occurrence of post-transplant complications is presented in Table 3. We did not find any significant differences in the incidence of PVT between Groups S and M or between Groups L and M (S vs. M vs. L = 1 [7.7%] vs. 5 [6.5%] vs. 0 [0%], pSM = 0.88, pML = 0.43). HAT and HVT only occurred in Group M, and the incidence in the groups did not differ to a statistically significant extent. Furthermore, the incidence of biliary complications in the two groups did not differ to a statistically significant extent. Gastrointestinal complications, mostly intestinal perforation, were significantly more common in Group S (7 of 13 patients, 53.9%) than in Group M (13 of 77 patients, 16.9%) (pSM < 0.01) (Table. 3).
The volume of ascites at 1 week post-transplantation was significantly higher in Group S than in the other groups (S vs. M vs. L = 1485.9 ± 1304.4 ml vs. 260.3 ± 446.9 ml vs. 138.6 ± 151.2 ml, pSM < 0.001, pML = 0.57). Still, there was no significant difference when the volume of ascites per body weight was compared (S vs. M vs. L = 26.5 ± 22.7 ml/kg vs. 22.2 ± 29.8 ml/kg vs. 24.0 ± 26.2 ml/kg, pSM = 0.63, pML = 0.87).
Regarding the postoperative biochemical and coagulation profiles, no obvious significant differences were observed in the postoperative trends of AST or PT–INR. Regarding ALT, recipients in Group S had a significantly higher value than those in the other groups at 1-month post-transplantation. This characteristic of Group S was also observed in the level of T-Bil, which remained significantly higher than that of Group M from 3 days to 3 weeks post-transplantation. On the other hand, in Group L, the T-Bil level was significantly higher than that of the other groups even before transplantation and remained higher for a while after transplantation. However, after 1 month, the value had decreased to a similar level to Group M (Fig. 2).
Discussion
In Japan, the number of transplants from deceased donors increased with the revision of the Transplant Law. However, deceased pediatric donors are still uncommon, resulting in a serious shortage of size-matched pediatric liver grafts [19]. Therefore, most liver transplantations are performed from living donors in Japan, and LDLT from adult donors is widely performed in pediatric recipients. Before performing LDLT, the graft volume is estimated from a preoperative contrast-enhanced CT scan of the donor and the appropriate type of graft is selected. For infant recipients, reduced LLS grafts or mono-segmental grafts may also be selected to optimize the graft to match the recipient's abdominal cavity or prevent vascular complications and abdominal compartment syndrome. On the other hand, for adolescent or adult recipients, the right or left lobe may be selected as the graft to minimize risk to both the donor and recipient and allow for safe transplantation while still providing adequate liver volume for the patient. The appropriate size of a graft is determined by an index calculated by comparing it to the recipient's body size, and GRWR is one of those indices.
It has been reported that the liver accounts for approximately 2–3% of the body weight of a healthy adult [7, 20], which is equivalent to the GRWR of a healthy adult. Several clinical studies have reported clinical information on the safe limit of graft size mismatch. In terms of reports regarding the lower limit of GRWR, Lee et al. reported that a GRWR of less than 0.8 was identified as a risk factor for graft loss [8] in a multivariate analysis. Similarly to this report, 0.8 is often considered to be the lower limit for low-risk GRWR. In contrast, Kusakabe et al. showed that graft survival in the GRWR > 0.6% to < 0.8% group was comparable to that in the GRWR < 0.8% group, suggesting that grafts can be safely transplanted with a GRWR of < 0.8% [12]. Alves et al. reported that patients with a MELD score of > 18 and a GRWR of < 0.8% have a high risk of graft loss at 3 months after transplantation [21], while Ben et al. reported that Child–Pugh A patients have a good prognosis with a GRWR of approximately 0.6% [13]. Based on these findings, graft size should be considered depending on the preoperative liver function. Moon et al. reported a significantly higher risk of graft loss in patients with a GRWR of < 0.8% and a donor of age > 44 years [22]. This suggests that donor age may also be another factor to consider when using smaller grafts. In this study, graft survival in Group S was similar to that in Group M and did not differ to a statistically significant extent. Considering the characteristics of the patients in Group S, grafts from young donors (mean donor age, 42 years) were used in patients with relatively preserved preoperative liver function (mean MELD score, 15.2). In two cases, the recipients' brothers were selected as donors for recipients who had reached adulthood, suggesting that such selection may have led to better outcomes.
One of the adverse effects of small grafts is a postoperative change called the small-for-size syndrome (SFSS). Although there is no consensus about the definition and pathogenesis of SFSS, we diagnosed it as protracted cholestasis, coagulopathy, and massive ascites in disparity with the normalization of transaminase levels, together with normal inflow and outflow on Doppler ultrasonography [13, 23]. Dahm et al. proposed “the presence of two of the following on three consecutive days: bilirubin > 100 umol/l (5.8 mg/dL), INR > 2, and encephalopathy grade 3 or 4.” as the definition of SFSS [6], and several papers have reported this accordingly [22, 24]. Furthermore, portal hyperperfusion has been suggested to be a causative factor of SFSS. Several animal studies have reported graft injuries caused by portal hyperperfusion. In a pig liver transplant model, small-for-size grafts showed frank rupture of the sinusoidal lining and parenchymal hemorrhage 1 h after reperfusion [25]. In another report, electron microscopic examination of grafts from rats undergoing liver transplantation with small-for-size grafts showed sinusoidal congestion, irregular large gaps of sinusoidal lining cells, and collapse of the space of Disse [26]. Similar effects of portal hyperperfusion have been reported in human grafts affected by SFSS, where changes such as the predominance of biliary stasis in the central lobular region, vacuolar injury of hepatocytes, ballooning degeneration, and punctate necrosis have been observed [27]. On the other hand, there have been reports of massive ascites suggesting SFSS even in patients with portal hyperperfusion even with grafts with a GRWR of ≥ 0.8 [28]. We have reported that some patients with a GRWR of > 0.8 could suffer from hyperbilirubinemia or persistent thrombocytopenia, depending on portal venous flow, and that the graft volume-to-splenic volume ratio is an important predictor [5].
Although it was necessary to adjust the portal blood flow of Group S by performing splenectomy in this study, the PVF/GV ratio was not significantly different from that of Group M. After these adjustments, there were no significant differences in patient or graft survival rates. In addition, there were no significant differences in ascites volume per body weight or liver enzymes after transplantation, and the only significant difference in postoperative changes was in T-Bil levels. This suggests that the incidence of SFSS, which is one of the greatest concerns in cases with a GRWR below the reference value, can be reduced if blood flow can be adequately adjusted. In this study, there were few cases with a GRWR of < 0.6%, and it was difficult to statistically determine a lower limit; however, the lack of major problems in Group S suggested that the lower limit of GRWR should be < 0.8%. In contrast, patients with high preoperative MELD scores had prolonged high T-Bil levels after transplantation, suggesting that large grafts should be aggressively considered in patients with a poor pretransplant liver function.
This study revealed that the operative time in Group S was significantly longer in comparison to the other groups. This was thought to be partly because many patients in Group S used the right lobe, which required a longer bench surgery for venoplasty. In addition, Group S, which included many cases of biliary atresia in which transplantation was performed in older children or adults, was expected to have strong intra-abdominal adhesions due to repeated cholangitis and other problems during the long-term course of their disease. The time required for detachment of these adhesions may have contributed to the longer operative time and higher rate of gastrointestinal tract perforation in comparison to the other groups, as we have previously reported [29].
While a large graft size is a relatively rare problem, it can still be encountered in neonatal and infantile transplants [14]. In a national survey reviewing reports on the maximum GRWR, Kasahara et al. [10] reported that the survival rate was poor in patients under 1 year of age who received grafts with a GRWR of > 4.0%. In contrast, Joao Seda Neto et al. [14] reported that grafts with a GRWR of > 4.0% had no negative effect on patient survival, while Marek Stefanowicz et al. [15] reported that a GRWR of up to 5.0% may be acceptable based on the results of a retrospective study of four groups divided by GRWR. One of the well-known secondary risks of oversizing is an increased risk of impaired perfusion, leading to vascular thrombosis [1]. Graft compression due to a small abdominal cavity volume can cause inadequate tissue perfusion [14]. Uchida et al. reported that the GRWR was significantly higher in patients with HAT than in those without HAT (HAT vs. non-HAT = 3.16% vs. 2.5% [median]) and that the GRWR was an independent risk factor for HAT [30]. Chang et al. [11] also reported that a GRWR of > 3.0 is a risk factor for PVT. Based on these reports, we used a GRWR of 3.5% as the cutoff value for grouping in this study.
Several other collateral risks of oversizing due to a small abdominal cavity volume are of concern in small children. Among them, ventilation problems and prolonged ventilation support have been reported. Levesque et al. reported that patients with a GRWR of > 2.5% experienced longer intubation management times and suggested that the use of large grafts may lead to cranial compression of the diaphragm, which may lead to airway closure and reduced lung capacity. The risk of abdominal compartment syndrome due to the small size of the recipient's abdominal cavity has been a concern for some time [7, 10]. Notably, it has been reported that transplant recipients with acute liver failure are more likely to experience exacerbations of intestinal edema during portal vein clamping due to the absence of portal collateral circulation, making delayed abdominal wall closure more likely to be considered [31]. A possible solution to these problems is delayed closure of the abdominal wall. Oikawa et al. reported a significantly higher GRWR in a group that underwent closure with a silicone sheet in comparison to their primary closure group. They suggested that if the GRWR is > 2.5%, special care should be taken to close the abdominal wall [32]. In this study, a significantly greater number of patients in Group L underwent delayed closure of the abdominal wall. There was no significant difference in the survival rates of Groups M and Ls, and there were no vascular complications in Group L. These results suggest that even if the GRWR is > 3.5%, blood flow to the graft may be maintained, and the prognosis may be good if delayed abdominal wall closure is considered in each case.
In this study, T-Bil was found to be significantly higher in Group L than in Group M up to 2 weeks after transplantation. We hypothesized that this was not due to graft size, but rather due to the fact that many patients in Group L had severe hepatic dysfunction that required transplantation at an early age and took a long time to recover after transplantation. This may also be because all patients in Group L received transplants with cholestatic disease as the primary disease.
The limitation of this single-center retrospective study is that the number of cases outside the reference range was relatively small. This makes it difficult to present and statistically verify the specific lower and upper limits of GRWR. A multicenter study with a larger cohort is needed to determine the reference values more precisely. Another limitation is that ligation of collateral vessels, splenectomy, or delayed abdominal wall closure is performed without objective indicators. We decide comprehensively whether to perform those procedure, considering the graft size, the portal vein blood flow velocity measured intraoperatively, or whether the graft is compressed by abdominal wall closure. Furthermore, it is difficult to say to what extent these procedures reduce the risk of complications. Further studies focusing on each technique’s outcome with objective indicators are warranted.
In conclusion, no significant difference was observed in graft survival in cases beyond the GRWR threshold. When the GRWR is < 0.8 or > 3.5, the risk of complications associated with graft size might be overcome with adjustments to provide appropriate portal blood flow or by considering delayed abdominal wall closure.
Data availability
No datasets were generated or analysed during the current study.
References
Wan P, Li Q, Zhang J, Shen C et al (2015) Influence of graft size matching on outcomes of infantile living donor liver transplantation. Pediatr Transplant 19(8):880–887. https://doi.org/10.1111/petr.12592. (Epub 2015 Sep 23)
Ogawa K, Kasahara M, Sakamoto S et al (2007) Living donor liver transplantation with reduced monosegments for neonates and small infants. Transplantation 83(10):1337–1340. https://doi.org/10.1097/01.tp.0000263340.82489.18
Kasahara M, Uryuhara K, Kaihara S et al (2003) Monosegmental living donor liver transplantation. Transplant Proc 35(4):1425–1426. https://doi.org/10.1016/s0041-1345(03)00445-7
Sugawara Y, Makuuchi M, Takayama T et al (2001) Small-for-size grafts in living-related liver transplantation. J Am Coll Surg 192(4):510–513. https://doi.org/10.1016/s1072-7515(01)00800-6
Takahashi Y, Matsuura T, Yoshimaru K et al (2018) Liver graft-to-spleen volume ratio as a useful predictive factor of the early graft function in children and young adults transplanted for biliary atresia: a retrospective study. Transpl Int 31(6):620–628. https://doi.org/10.1111/tri.13131. (Epub 2018 Feb 23)
Dahm F, Georgiev P, Clavien PA (2005) Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. https://doi.org/10.1111/j.1600-6143.2005.01081.x
Kiuchi T, Kasahara M, Uryuhara K et al (1999) Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 67(2):321–327. https://doi.org/10.1097/00007890-199901270-00024
Lee DS, Gil WH, Lee HH et al (2004) Factors affecting graft survival after living donor liver transplantation. Transplant Proc 36(8):2255–2256. https://doi.org/10.1016/j.transproceed.2004.08.073
Levesque E, Duclos J, Ciacio O, Adam R, Castaing D, Vibert E (2013) Influence of larger graft weight to recipient weight on the post-liver transplantation course. Clin Transplant. https://doi.org/10.1111/ctr.12059. (Epub 2013 Jan 7)
Kasahara M, Sakamoto S, Umeshita K et al (2014) Effect of graft size matching on pediatric living-donor liver transplantation in Japan. Exp Clin Transplant 12(Suppl 1):1–4. https://doi.org/10.6002/ect.25liver.l5
Cheng YF, Chen CL, Huang TL et al (2004) Risk factors for intraoperative portal vein thrombosis in pediatric living donor liver transplantation. Clin Transplant 18(4):390–394. https://doi.org/10.1111/j.1399-0012.2004.00178.x
Kusakabe J, Yagi S, Sasaki K et al (2021) Is 0.6% Reasonable as the minimum requirement of the graft-to-recipient weight ratio regardless of lobe selection in adult living donor liver transplantation? Transplantation 105(9):2007–2017. https://doi.org/10.1097/TP.0000000000003472
Ben-Haim M, Emre S, Fishbein TM et al (2001) Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient’s disease. Liver Transpl 7(11):948–953. https://doi.org/10.1053/jlts.2001.29033
Neto JS, Carone E, Pugliese V, Salzedas A et al (2007) Living donor liver transplantation for children in Brazil weighing less than 10 kilograms. Liver Transpl 13(8):1153–1158. https://doi.org/10.1002/lt.21206
Stefanowicz M, Kowalewski G, Szymczak M et al (2021) Impact of graft size matching on the early post-transplant complications and patients survival in children after living donor liver transplantations. Children (Basel) 8(7):579. https://doi.org/10.3390/children8070579
Urata K, Kawasaki S, Matsunami H et al (1995) Calculation of child and adult standard liver volume for liver transplantation. Hepatology 21(5):1317–1321
Kamath PS, Wiesner RH, Malinchoc M et al (2001) A model to predict survival in patients with end-stage liver disease. Hepatology 33:464–470. https://doi.org/10.1053/jhep.2001.22172
McDiarmid SV, Anand R, Lindblad AS (2002) Principal investigators and institutions of the studies of pediatric liver transplantation (SPLIT) research group Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation 74:173–181. https://doi.org/10.1097/00007890-200207270-00006
The Japan Society of Transplantation. http://www.asas.or.jp/jst/general/number/
Tanaka G, Nakahara Y, Nakazima Y (1989) Studies on the weight and size of internal organs of Normal Japanese. Nippon Igaku Hoshasen Gakkai Zasshi 49(3):344–364
Alves RC, Fonseca EA, Mattos CA et al (2012) Predictive factors of early graft loss in living donor liver transplantation. Arq Gastroenterol 49(2):157–161. https://doi.org/10.1590/s0004-28032012000200011
Moon JI, Kwon CH, Joh JW et al (2010) Safety of small-for-size grafts in adult-to-adult living donor liver transplantation using the right lobe. Liver Transpl 16(7):864–869. https://doi.org/10.1002/lt.22094
Suehiro T, Shimada M, Kishikawa K et al (2005) Effect of intraportal infusion to improve small for size graft injury in living donor adult liver transplantation. Transpl Int 18(8):923–928. https://doi.org/10.1111/j.1432-2277.2005.00159.x
Ikegami T, Kim JM, Jung DH et al (2019) Conceptual changes in small-for-size graft and small-for-size syndrome in living donor liver transplantation. Korean J Transplant. https://doi.org/10.4285/jkstn.2019.33.4.65
Kelly DM, Demetris AJ, Fung JJ et al (2004) Porcine partial liver transplantation: a novel model of the “small-for-size” liver graft. Liver Transplant. https://doi.org/10.1002/lt.20073
Man K, Lo CM, Ng IO et al (2001) Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surgery. https://doi.org/10.1001/archsurg.136.3.280
Emond JC, Renz JF, Ferrell LD et al (1996) Functional analysis of grafts from living donors Implications for the treatment of older recipients. Ann Surg 224(4):544–552. https://doi.org/10.1097/00000658-199610000-00012. (discussion 552-4)
Shirouzu Y, Ohya Y, Suda H et al (2010) Massive ascites after living donor liver transplantation with a right lobe graft larger than 0.8% of the recipient’s body weight. Clin Transplant 24(4):520–527. https://doi.org/10.1111/j.1399-0012.2009.01117.x
Yanagi Y, Matsuura T, Hayashida M et al (2017) Bowel perforation after liver transplantation for biliary atresia: a retrospective study of care in the transition from children to adulthood. Pediatr Surg Int 33:155–163. https://doi.org/10.1007/s00383-016-4008-9
Uchida Y, Sakamoto S, Egawa H et al (2009) The impact of meticulous management for hepatic artery thrombosis on long-term outcome after pediatric living donor liver transplantation. Clin Transplant 23(3):392–399. https://doi.org/10.1111/j.1399-0012.2008.00924.x. (Epub 2008 Dec 16)
Khorsandi SE (2017) Is size the only determinant of delayed abdominal closure in pediatric liver transplant? Liver Transplant. https://doi.org/10.1002/lt.24712
Oikawa K, Ohkohchi N, Kato H et al (1998) Graft weight/recipient body weight ratio (G/R ratio) in living-related liver transplantation for pediatric patients: abdominal wall closure in cases with a large G/R ratio. Transplant Proc 30(7):3209–3210. https://doi.org/10.1016/s0041-1345(98)00998-1
Acknowledgements
We thank Dr. Brian Quinn for editing the English language of this manuscript.
Funding
The authors declare no funding.
Author information
Authors and Affiliations
Contributions
Y.T. designed the research/study, performed the research/study, analyzed the data, and wrote the paper. T.M. designed the research/study and revised the manuscript. Y.T., Y.U., K.K., and S.M. collected data. N.K., K.N., and T.T. revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toriigahara, Y., Matsuura, T., Takahashi, Y. et al. A retrospective study investigating the risk of graft loss in living donor liver transplant cases where size mismatching is predicted from graft-to-recipient weight ratio. Pediatr Surg Int 40, 229 (2024). https://doi.org/10.1007/s00383-024-05814-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-024-05814-w