Abstract
Purpose
Megacystis–microcolon–intestinal hypoperistalsis syndrome (MMIHS) is a well described clinical condition, but reports are focused on microcolon and intestinal hypoperistalsis, while data on bladder management are scant.
Aim of the study is to present urological concerns in MMIHS.
Methods
Retrospective evaluation of clinical data on urological management of MMIHS patients treated in the last 10 years.
Results
Six patients were enrolled (3 male, 3 female). Three girls had prenatal diagnosis of megacystis (1 vesicoamniotic shunt was placed). All patients had genetic diagnosis: 5 had ACTG2 gene mutations and 1 MYH11 mutation. All patients were addressed to our attention for urinary symptoms, such as urinary retention, urinary tract infections, acute renal injury. Two patients presented frequent stoma prolapses. All children underwent a complete urological evaluation, and then started a bladder management protocol (clean intermittent catheterization, via urethra or cystostomy-tube placement), with improvement of urinary infections, upper urinary tract dilation and stoma prolapses, if present. All patients had good renal function at last follow-up.
Conclusion
We believe that MMIHS patients must be addressed soon and before onset of symptoms for a multidisciplinary evaluation, including an early assessment by a pediatric urologist expert in functional disorder, to preserve renal function at its best.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Megacystis–microcolon–intestinal hypoperistalsis syndrome (MMIHS) is a rare disease characterized by high morbidity and mortality. It is marked by megacystis (bladder distention without mechanical obstruction), microcolon, and intestinal hypoperistalsis [1].
Typically diagnosed postnatally, due to bowel and/or bladder obstruction symptoms, it can be suspected prenatally in 26% of patients mainly when megacystis is present, thanks to genitourinary and/or gastrointestinal findings, especially in case of positive family history [2]. Early symptoms include urinary retention, abdominal distension, bilious vomiting, and failure to pass meconium, even if clinical manifestation can vary widely. Due to frequent episodes of intestinal non-mechanical obstructions, MMIHS is a known cause of pediatric intestinal pseudo-obstruction (PIPO), defined as a chronic inability (persisting for 2 months from birth or ≥ 6 months thereafter) of gastrointestinal system to propel its contents, in absence of mechanical obstruction. These children often require multiple intestinal surgeries, potentially leading to a short bowel syndrome, and necessitating different gastro/intestinal derivations (e.g., gastrostomy, jejunostomy, ileostomy). Patients with MMIHS need life-long care, often including central venous catheter (CVC) placement for total parenteral nutrition, with risk of hepatic insufficiency and CVC-related sepsis [1, 3,4,5,6,7]. Because of the prominence of bowel symptoms, bladder dysfunction could be overlooked, with an increased risk of vesico-ureteral reflux (VUR), hydronephrosis, urinary infection and chronic renal failure.
Given the scarcity of data on the urological management of MMIHS patients, we decided to perform a retrospective analysis of our experience of the last 10 years with MMIHS, focusing particularly on bladder care, to define possible concerns on diagnosis and urological management.
Methods
We retrospectively reviewed charts of MMIHS patients referred to our Pediatric Urology Division between September 2013 and September 2023. Written consent has been obtained by parents, according to local law.
Our institution houses several multidisciplinary clinical teams dedicated to the long-term care of rare and complex surgical diseases but a specialized team for MMIHS was established only 3 years ago. In the case of MMIHS, all patients underwent a comprehensive evaluation by pediatrician, gastroenterologist, nutritionist, geneticist, physiatrist, digestive surgeon and pediatric urologist.
We included all patients with MMIHS diagnosis confirmed by genetic analysis.
We focused on the diagnostic tests and the surgical procedures performed, particularly on the urological aspects.
Results
During the 10-year study period, 6 MMIHS were referred to our division: 3 males and 3 females.
All patients had genetic diagnosis: 5 ACTG2 mutations and 1 MYH11 mutation. The three females had prenatal diagnosis of megacystis. Among these girls, one had prenatal detection of ACTG2 mutation during amniocentesis; she had vesicoamniotic shunt placement (Fig. 1), which was removed after birth.
The age at our hospital referral varied from birth to ten months. All patients underwent intestinal surgeries and subsequently required management of bladder emptying. The median age at our first evaluation was 2 years (interquartile: birth–5.5 years).
All patients had urinary retention as the first urological symptom. Urinary tract infections (UTIs) were common, occurring in 5/6 (83%) patients; these infections were no longer observed after adequate bladder management. Upper urinary tract (UUT) dilations were found in 4/6 (67%) patients before the initiation of bladder emptying management.
Two patients (1 male and 1 female) experienced acute kidney injury (AKI), successfully treated with suprapubic tube placement. The female patient had AKI during her 1st month of life; she underwent suprapubic catheter placement, which was kept in place for 5 months, then her caregivers were trained to start CIC. The male patient presented AKI, with hematuria and urinary retention as a manifestation of urological onset, at 7 years old, after colectomy surgery; he categorically refused catheterization via urethra, so a suprapubic tube was placed, with positive clinical outcome; the tube was then substituted with a button cystostomy [8].
Button cystostomy [8] was placed in 4/6 patients: 3 males because of categorical refusal of CIC via urethra (due to sensate urethra) and 1 female. She was on CIC via urethra since the 1st month of life (because of episodes of urinary retention) but several febrile UTIs occurred and radiological images of megacystis persisted; a button cystostomy was placed with no longer febrile UTIs; after its placement we recorded also a reduction of intestinal obstructive episodes.
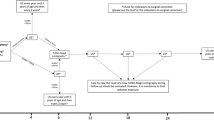
In two patients we observed resolution of the frequent episodes of ileostomy prolapse following bladder management. One patient improved after permanent catheterization at 4 years old, so we decided to place a button cystostomy (CIC via urethra was not well tolerated, as already mentioned) to allow emptying every 2–3 h and continuous drainage into a collection bag overnight. The other patient had a cystostomy creation at birth in another hospital; a program of stoma catheterization was started and then abandoned (poor compliance). When he was referred to our division, at 6 years of life, the cystostomy was not catheterizable anymore, he had UTIs and urinary retention episodes with ileostomy prolapses (Fig. 2); so, we decided to close the cystostomy and place a suprapubic button cystostomy [8] (Fig. 3).
Median follow-up was 2 years (interquartile 1–6.75 years).
At the last multidisciplinary follow-up, all patients were in good overall condition; 4 patients had cystostomy, 2 were on CIC via urethra; all had normal renal function. Figure 4 shows stomas disposition at last follow-up in a male patient.
Tables 1 and 2 summarize patient characteristics, described in detail below.
Discussion
MMIHS is a rare disease with female predominance (female-to-male ratio, 2.1:1), first described in 1976 in 5 female newborns presenting with abdominal distension, caused by giant bladder and intestinal non-mechanical obstruction. Almost 450 cases were described since then [2, 9].
MMIHS is characterized by significant morbidity and mortality. Gosemann et al. reported in 2011 a survival rate of 19.7% (43/218 patients) at the time of their publication. In 2014 Tuzoric et al. reported a decrease in mortality with survival rate exceeding 50%. Prathapan et al. reported in 2021 an estimated survival rate of 100%, 100% and 86% at 5-, 10- and 20-year follow-up, respectively. The major causes of death include sepsis (due to intestinal overgrowth, post-operative and central-catheter infections), malnutrition, parenteral nutrition-associated liver disease, chronic kidney disease, and multiple organ failure. Currently, early diagnosis, advances in intestinal rehabilitation and transplantation, in addiction to an early referral of these patients to tertiary care center, with multidisciplinary approach that includes a pediatric urologist, have increased the survival rate [2, 7, 9, 10].
The complete etiopathogenesis of MMIHS is not yet fully understood, although several hypotheses have been proposed: genetic, neurogenic, myogenic, and hormonal. However, several cases remain with undefined genetic origin [1, 3, 9, 11, 12]. Among our patients, all received a genetic diagnosis: five had ACTG2 mutation and one had MYH11 mutation. Diagnosis can occur prenatally or usually within the first year of life. Prenatal diagnosis remains challenging, due to the lack of specificity of the findings, and it could be made during the second (70%) or third (23%) trimester. The most frequent prenatal ultrasound finding is megacystis, which is defined by the longitudinal diameter of the bladder ≥ 7 mm during first trimester; for the subsequent periods, there are no clear defining parameters [9, 13, 14]. Tuzovic et al. reported a prenatal detection of MMIHS in 13/50 newborns (26% of cases); of these, 7 had a sibling affected of the same pathology. Prenatal US usually reveals urinary impairment, with finding of megacystis with or without hydroureteronephrosis (88%) or isolated hydronephrosis (10%); other findings may include hydroureter, the “keyhole” sign, dilated bowel loops with or without gastric distension. Amniotic fluid level can be normal (60%), increased (27%) or reduced (4%). Some authors suggest fetal magnetic resonance (MR), to better define the fetal anatomy. The most common prenatal invasive procedure is amniocentesis. Other fetal procedures performed in these patients are vesicocentesis, vesicoamniotic shunting and fetal urinalysis [1, 2, 7, 10, 13]. Among our patients, the 3 females had prenatal diagnosis of megacystis; one of them also underwent fetal positioning of vesicoamniotic shunt and amniocentesis, showing ACTG2 mutation. One male patient exhibited left hydronephrosis on prenatal US, no longer detected during postnatal follow-up.
After birth, newborns with MMIHS usually present an abdominal distension in a scenario of intestinal and bladder non-mechanical obstruction. However, clinical manifestation can vary greatly, with variable severity, potentially complicating the diagnosis. Early urinary non-obstructive retention combined with abdominal distension or intestinal obstruction may lead to a suspicion of MMIHS [1, 3,4,5,6,7].
Due to the prevalence of gastrointestinal symptoms, urinary tract studies are often performed during abdominal US examinations. VCUG could be useful, showing a dilated atonic bladder, with or without VUR and elevated post-voidal residual. In Literature, VUR is rarely described in these patients (approximately 4%), although Hugar et al. reported higher rates of associated VUR in their cohort of MMIHS patients, with one of them endoscopically treated with Deflux. None of our patients had cystographic diagnosis of VUR. Urodynamic study can also be effective in demonstrating increased bladder capacity and compliance, detrusor hypocontractility/acontractility and ineffective emptying. However, there is limited data in literature on urodynamic evaluations of these patients. Sacral US and Spine MR can be aid in early detection of sacral anomalies, a possible cause of neurogenic bladder and bowel dysfunction [2, 4, 6, 7]. Regarding urological management, CIC is a mainstay, usually required life-long to improve bladder emptying and prevent UTI, UUT dilation and renal damage. We also noted a reduction in the frequency and/or severity of ileostomy prolapse episodes in patients who underwent correct bladder emptying. Different gastrointestinal stomas are usually performed in these patients, due to the frequent and severe episodes of obstruction; stoma-related morbidity is common in patients with MMIHS. Irtan et al. found stoma prolapse in 6/23 patients (26%) with jejunostomy and 4/14 children (29%) with cecocolostomy. Some of these prolapses required reduction under general anesthesia, other needed surgical correction [15].
Unfortunately, patients with MMIHS are not always managed by pediatric urologist at the time of MMIHS diagnosis.
Different factors may contribute to this missing approach. In the same instance, this may be due to the limited knowledge about urological outcomes and therapeutic strategies for these complex patients. In others, the focus may be on the more dramatic intestinal symptoms, or there might be a delay in recognizing bladder dysfunction. Moreover, the high morbidity of these patients, characterized by numerous hospital admissions, can obscure the underlying bladder dysfunction, which can be attributed to psychological comorbidities [16]. Hugar et al. [7] reported a 20% (5/26) of patients were able to void voluntarily or had no bladder management regimen at the time of presentation to their unit. They found a poor prognosis for sustained spontaneous voiding. Given the above data, the urological approach is not standardized. Some authors suggest initiating CIC soon after birth, to foster a greater acceptance of catheterization by patients and their families, leaving the creation of a vesicostomy in case of CIC failure. Indeed, CIC may not be acceptable to these patients, especially boys, due to a sensate urethra. Other bladder drainage methods include suprapubic tube placement, continent vesicostomy or catheterizable conduit, such as Mitrofanoff appendicovesicostomy. In a systematic review, Gosemann et al. reported vesicostomy creation in 41/227 patients [10]. In our experience CIC is the first line management, to start as soon as possible, based on US evaluation of upper and lower urinary tract [17]. A button cystostomy is a feasible, safe and effective option, especially in male patients with sensate urethra and refusal of urethral catheterism. In addition to bladder derivation, further surgical interventions could be necessary, such as treatment for bladder or kidney stones. Regarding recurrent UTIs, common in these patients, some authors suggest daily antibiotic prophylaxis, albeit with increased risk of multidrug resistant organism [4, 7, 8, 10, 18]. We believe that bladder irrigation with gentamicin or another antibiotic could be an alternative.
In our experience, adequate management of bladder dysfunction can also be beneficial for bowel management. We believe that a proper bladder emptying can reduce intrabdominal pressure, leading to a decrease in the incidence of intestinal stomas prolapses. This aspect has not been previously described and no data are reported on any significant risk factor that favor prolapse in MMIHS patients, potentially due to the increased abdominal pressure caused by megacystis [4, 10, 15, 18].
Limits of our study: Our results need to be confirmed by a large cohort; other study’s limits are the retrospective nature of our evaluation, short-term follow-up, and single-center experience.
Conclusions
We believe that the urological evaluation is necessary soon after birth, to start promptly bladder management and urological follow-up. A standardized protocol for urological study and treatment should be established, to achieve better intestinal and urological outcome. A large multicentric study could be useful to create a standardized urological management protocol for these complex patients.
Data availability
Patients’ data are reported in this paper. Other data are available in their clinical charts if necessary.
References
Ambartsumyan L (2019) Megacystis-microcolon-intestinal hypoperistalsis syndrome overview. In: Adam MP, Mirzaa GM, Pagon RA et al (eds) GeneReviews® [Internet]. University of Washington, Seattle, pp 1993–2023
Tuzovic L, Anyane-Yeboa K, Mills A, Glassberg K, Miller R (2014) Megacystis-microcolon-intestinal hypoperistalsis syndrome: case report and review of prenatal ultrasonographic findings. Fetal Diagn Ther 36(1):74–80. https://doi.org/10.1159/000357703. (Epub 2014 Feb 21 PMID: 24577413)
Milunsky A, Baldwin C, Zhang X, Primack D, Curnow A, Milunsky J (2017) Diagnosis of chronic intestinal pseudo-obstruction and megacystis by sequencing the ACTG2 gene. J Pediatr Gastroenterol Nutr 65(4):384–387. https://doi.org/10.1097/MPG.0000000000001608. (PMID:28422808;PMCID:PMC5610062)
Wymer KM, Anderson BB, Wilkens AA, Gundeti MS (2016) Megacystis microcolon intestinal hypoperistalsis syndrome: case series and updated review of the literature with an emphasis on urologic management. J Pediatr Surg 51(9):1565–1573. https://doi.org/10.1016/j.jpedsurg.2016.06.011. (Epub 2016 Jun 26 PMID: 27421821)
Mori M, Clause AR, Truxal K, Hagelstrom RT, Manickam K, Kaler SG, Prasad V, Windster J, Alves MM, Di Lorenzo C (2022) Autosomal recessive ACTG2-related visceral myopathy in brothers. JPGN Rep 3(4):e258. https://doi.org/10.1097/PG9.0000000000000258.PMID:37168481;PMCID:PMC10158422
Thapar N, Saliakellis E, Benninga MA, Borrelli O, Curry J, Faure C, De Giorgio R, Gupte G, Knowles CH, Staiano A, Vandenplas Y, Di Lorenzo C (2018) Paediatric intestinal pseudo-obstruction: evidence and consensus-based recommendations from an ESPGHAN-Led Expert Group. J Pediatr Gastroenterol Nutr 66(6):991–1019. https://doi.org/10.1097/MPG.0000000000001982. (PMID: 29570554)
Hugar LA, Chaudhry R, Fuller TW, Cannon GM, Schneck FX, Ost MC, Stephany HA (2018) Urologic phenotype and patterns of care in patients with megacystis microcolon intestinal hypoperistalsis syndrome presenting to a major pediatric transplantation center. Urology 119:127–132. https://doi.org/10.1016/j.urology.2018.05.002. (Epub 2018 May 9 PMID: 29752972)
Mosiello G, Lopes Mendes AL, Capitanucci ML, Zaccara AM, De Gennaro M (2017) Button cystostomy: is it really a safe and effective therapeutic option in pediatric patients with neurogenic bladder? Urology 101:73–79. https://doi.org/10.1016/j.urology.2016.09.025. (Epub 2016 Sep 29 PMID: 27693876)
Prathapan KM, King DE, Raghu VK, Ackerman K, Presel T, Yaworski JA, Ganoza A, Bond G, Sevilla WMA, Rudolph JA, Alissa F (2021) Megacystis microcolon intestinal hypoperistalsis syndrome: a case series with long-term follow-up and prolonged survival. J Pediatr Gastroenterol Nutr 72(4):e81–e85. https://doi.org/10.1097/MPG.0000000000003008.PMID:33264186;PMCID:PMC9124153
Gosemann JH, Puri P (2011) Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int 27(10):1041–1046. https://doi.org/10.1007/s00383-011-2954-9. (PMID: 21792650)
Nakamura H, O’Donnell AM, Puri P (2019) Consanguinity and its relevance for the incidence of megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS): systematic review. Pediatr Surg Int 35(2):175–180. https://doi.org/10.1007/s00383-018-4390-6. (Epub 2018 Nov 1 PMID: 30386895)
Matera I, Bordo D, Di Duca M, Lerone M, Santamaria G, Pongiglione M, Lezo A, Diamanti A, Spagnuolo MI, Pini Prato A, Alberti D, Mattioli G, Gandullia P, Ceccherini I (2021) Novel ACTG2 variants disclose allelic heterogeneity and bi-allelic inheritance in pediatric chronic intestinal pseudo-obstruction. Clin Genet 99(3):430–436. https://doi.org/10.1111/cge.13895. (Epub 2020 Dec 14 PMID: 33294969)
Fontanella F, Maggio L, Verheij JBGM, Duin LK, Adama Van Scheltema PN, Cohen-Overbeek TE, Pajkrt E, Bekker M, Willekes C, Bax CJ, Gracchi V, Oepkes D, Bilardo CM (2019) Fetal megacystis: a lot more than LUTO. Ultrasound Obstet Gynecol 53(6):779–787. https://doi.org/10.1002/uog.19182. PMID: 30043466; PMCID: PMC6593717
Capone V, Persico N, Berrettini A et al (2022) Definition, diagnosis and management of fetal lower urinary tract obstruction: consensus of the ERKNet CAKUT-Obstructive Uropathy Work Group. Nat Rev Urol 19:295–303. https://doi.org/10.1038/s41585-022-00563-8
Irtan S, Bellaïche M, Brasher C, El Ghoneimi A, Cézard JP, Bonnard A (2010) Stomal prolapse in children with chronic intestinal pseudoobstruction: a frequent complication? J Pediatr Surg 45(11):2234–2237. https://doi.org/10.1016/j.jpedsurg.2010.06.022. (PMID: 21034950)
von Gontard A, Vrijens D, Selai C, ... Apostolidis A, Anding R (2019) Are psychological comorbidities important in the aetiology of lower urinary tract dysfunction – ICI-RS 2018? Neurourol Urodyn 38(S5):S8–S17
De Gennaro M, Capitanucci ML, Di Ciommo V, ... Orazi C, Tubaro A (2006) Reliability of bladder volume measurement with BladderScan® in paediatric patients. Scand J Urol Nephrol 40(5):370–375
Grosman J, Aigrain Y, Goulet O, Lacaille F, Capito C, Chardot C (2022) Preservation of native sigmoid colon for secondary continent cystostomy after multivisceral transplantation for chronic intestinal pseudo-obstruction. Pediatr Transplant 26(2):e14180. https://doi.org/10.1111/petr.14180. Epub 2021 Nov 8. PMID: 34747091
Acknowledgements
This work is generated within the European Reference Network for Rare Urogenital Diseases and Complex Conditions (ERN EUROGEN).
Funding
This work was supported also by the Italian Ministry of Health with Current Research funds. This manuscript has been viewed and approved by all authors.
Author information
Authors and Affiliations
Contributions
Conceptualization: CP, GM; methodology: MC, FS; formal analysis and investigation: AZ, RT, GB, MS; writing—original draft preparation: FF, PDA, BI, VF; writing—review and editing: AD, AC, TC; supervision: GM, MC.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pellegrino, C., Barone, G., Capitanucci, M.L. et al. Megacystis–microcolon–intestinal hypoperistalsis syndrome: don’t forget the bladder. Pediatr Surg Int 40, 124 (2024). https://doi.org/10.1007/s00383-024-05711-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-024-05711-2