Abstract
Purpose
Immunological abnormalities have been hypothesized as a pathogenesis of biliary atresia (BA). We previously investigated the frequency and function of circulating regulatory T-cells (Tregs) and reported no differences compared to controls. However, the local Treg profile remains uncertain. We aimed to investigate the frequency of Tregs in BA liver tissues.
Methods
The number of lymphocytes, CD4+ cells, and CD4+FOXP3+ Tregs infiltrating the portal tract and the percentage of Tregs among CD4+ cells of BA and control patients were visually counted. The correlation between these data and clinical indicators was also examined.
Results
The number of lymphocytes, CD4+ cells, and CD4+FOXP3+ Tregs was higher in the BA group. However, the percentage of Tregs among CD4+ cells was similar in both groups. Each parameter was correlated with serum γ-GTP, but there was no clear association with liver fibrosis, jaundice clearance, and native liver survival.
Conclusion
The number of Tregs infiltrating the portal tract was higher in BA patients. However, the infiltration of lymphocytes was also generally increased. Tregs appear to be unsuccessful in suppressing progressive inflammation in BA patients, despite recruitment to local sites. Investigation of Treg function in the local environment is warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biliary atresia (BA) is a severe neonatal liver disease characterized by an inflammatory and fibrotic biliary obstruction causing cholestasis and progressive liver failure [1,2,3]. Although BA is a rare disease, the incidence of which has been reported as one in 8000–15,000 live births, it is the most common cause of neonatal cholestasis [4, 5]. Even in cases with successful hepatoportoenterostomy, more than half of patients eventually develop cirrhosis and require liver transplantation before adulthood. The etiology and pathogenesis of BA remain unclear, and various problems also exist in managing postoperative patients [1, 5,6,7,8]. Multifactorial etiological factors with developmental and environmental origins contribute to disease development. These factors include viruses, toxins, environmental exposures, developmental pathology, genetic abnormalities, aberrant neonatal immune responses, and abnormal fibrogenesis [1, 9, 10]. A leading hypothesis in the pathogenesis of BA is that unknown factors initiate bile duct injury within the perinatal period (e.g., virus, toxins, vascular insults), followed by an exaggerated inflammatory or autoimmune response targeting the bile duct epithelia, resulting in progressive bile duct injury and obliteration, and subsequently, secondary biliary cirrhosis [1, 6].
Autoimmune diseases develop from either a primary breakdown in tolerance or a breakdown secondary to a deficiency in immune regulation [1]. Regulatory T-cells (Tregs) are the key population of lymphocytes central to self-tolerance regulation and tissue homeostasis maintenance [11]. Treg defects have been shown to contribute to the loss of tolerance in human autoimmune diseases. Earlier studies have reported evidence supporting a role for numerical and functional Treg impairments in systemic and organ-specific autoimmune disorders, including autoimmune liver disease [12].
We recently investigated the circulating Tregs of BA patients and found no significant differences in Treg frequency and function compared to the control group. However, these results do not necessarily exclude the possibility that abnormal Treg frequency and function at the local site (e.g., liver, portal tract) play critical roles in the pathogenesis of BA. It is known that the number of Tregs is generally increased in the inflammation sites of an organ-specific autoimmune disease but is not significantly altered in the peripheral blood [13].
There is controversy concerning the population of Tregs infiltrating the hepatic system in BA. Some studies have shown the decreased frequency and function of Treg cells in the liver parenchyma using BA mouse models [14,15,16,17]. In contrast, the mRNA expression level of forkhead box protein 3 (Foxp3), a specific marker of Treg, was reported to be increased in BA liver in other studies [14, 18, 19], and an immunohistochemical analysis showed a decreased Foxp3-positive Treg cell percentage in one study [18] using human BA specimens. To date, only one published study has evaluated Treg infiltration to the portal tract. In this study, Treg was assessed by histological techniques using human specimens, and patients with non-cholestatic diseases, such as duodenal atresia/stenosis, were used as controls. It is known that bile acid metabolites promote the generation and differentiation of Treg cells [20, 21] and that bile duct ligation results in an expansion of liver Tregs [22]. Thus, cholestasis in BA patients may influence Treg frequency. Patients with non-cholestatic diseases may not be suitable as controls, and a study using patients with cholestatic diseases as controls is ideal.
In the present study, we aimed to investigate the frequency of local Tregs in BA patients compared to patients with other underlying cholestatic diseases and to evaluate the significance of Tregs in the pathogenesis of BA.
Patients and methods
Patients and samples
Liver tissue sampled by wedge biopsy was collected from eight BA patients and seven age-matched control cases. The control group consisted of four cases with suspected BA who had undergone cholangiography via laparotomy, two cases of congenital biliary dilatation (CBD), and one case with temporary liver damage after strangulated small bowel obstruction. Wedge biopsy was performed during hepatoportoenterostomy in BA patients, laparotomy for cholangiography in suspected BA, hepaticojejunostomy in CBD, and stoma closure in small bowel obstruction. We also collected the clinical parameters, namely, serum total bilirubin (T-bil), direct bilirubin (D-bil), alanine aminotransferase (ALT), and gamma glutamyl transferase P (γ-GTP) levels at the preoperative date closest to the date of sample collection, achievement/non-achievement of postoperative jaundice clearance, and histological liver fibrosis evaluation of each patient.
Histopathological findings
Lymphocyte counting and evaluation of liver fibrosis were performed by a single pathologist with hematoxylin and eosin (HE) staining. Lymphocytes infiltrating the portal tract were counted per 10,000 μm2. Liver fibrosis was evaluated according to the fibrosis score developed for BA as previously reported [23], in which fibrosis was defined as follows: mild (I), fibrosis ranging from portal fibrous expansion to bridging fibrosis involving < 50% of portal tracts; moderate (II), bridging fibrosis with > 50% of portal tracts involved without nodular architecture; and severe (III), bridging fibrosis with > 50% of portal tracts involved and nodular architecture.
Immunohistological staining
Liver tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin wax. After the sections had been deparaffinized in xylene and rehydrated in a graded ethanol series, they were immersed in EDTA solution (pH 8.0) and heated in a pressure cooker for antigen retrieval in the staining of FOXP3. For the CD4 staining, the sections were immersed in EDTA solution (pH 8.0), irradiated in a microwave oven for 5 min, and then allowed to cool down at room temperature for 20 min. The primary antibodies used were anti-FOXP3 (Abcam, ab20034) and anti-CD4 (Invitrogen 14-2444-82). Following the reaction of the primary antibodies, the samples were incubated with horseradish peroxidase (HRP)–labeled secondary antibodies (Nichirei). Double immunostaining was performed to identify the colocalization of FOXP3 and CD4. First, the sections were stained with anti–FOXP3 and visualized with DAB as described above. After the sections were rinsed with phosphate-buffered saline, they were heated in a microwave for antigen retrieval. Anti-CD4 antibody was used as the second antibody and visualized with HistoGreen (Linaris). Photomicrographs were taken with a BZ-X710 (KEYENCE CORPORATION, Osaka, Japan) microscope. For the semi-quantitative evaluation of the immunohistochemistry, five representative portal tracts containing interlobular bile ducts were chosen randomly in each section.
Statistical analysis
Statistical analysis was performed using JMP® Pro 16.0.0 software (SAS, Cary, NC, USA). The differences between the two groups were analyzed by Fisher’s exact test, the Wilcoxon rank-sum test, or Student’s-T test. The correlations for continuous variables were evaluated using Spearman’s rank correlation coefficient. A p-value of less than 0.05 was considered statistically significant.
Compliance with ethical standards
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Graduate School of Medicine, Chiba University (approval number 908) and the Institutional Review Board of Chiba Children’s Hospital (2017–046). Samples were taken as clinically necessary and used for the study after informed consent from the patient’s family was obtained.
Results
Patient characteristics
Table 1 shows the patients’ demographic data, including the sample collection timing and laboratory parameters of the BA and non-BA groups.
There was no significant difference in the female:male ratio (BA: 5:3; control: 2:5) (Fisher’s exact test, p = 0.31). The BA and non-BA groups were similar in age at the time of specimen collection (median [minimum—maximum]: BA: 43.5 [20–128] days; non-BA: 80 [30–157]; p = 0.46) serum ALT levels (BA: 59 [33–133] U/l; non-BA: 58 [19–112]; p = 0.63). Serum T-Bil (BA: 6.45 [4.4–15.2] mg/dl; non-BA: 3.5 [0.2–7.4]; p < 0.01), D-Bil (BA: 3.7 [2.6–11.1] mg/dl; non-BA: 2.5 [0–4.5]; p < 0.05), and γ-GTP (BA: 486 [207–633] U/L; non-BA: 165 [17–613]; p < 0.05) levels were significantly lower in the non-BA group (Table 1). The BA group included one case of prenatally detected cystic biliary atresia and seven cases of isolated type. There were no patients with CMV-IgM+-associated biliary atresia or biliary atresia splenic malformation syndrome.
Frequency of infiltrating CD4+ T cells and CD4+FOXP3+ Tregs in BA group and non-BA group
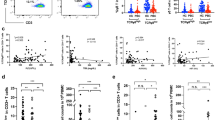
First, we counted the lymphocytes infiltrating the portal tract (Fig. 1). The number of lymphocytes per 10,000 μm2 was significantly higher in the BA group compared to the non-BA group (mean ± standard deviation [SD]: BA: 36 ± 12; non-BA: 22 ± 9.5; p < 0.05). Next, we counted the number of CD4+ cells and CD4+FOXP3+ cells (Tregs) infiltrating the portal tract (Fig. 2a, b). Both CD4+ T cells (BA: 26 ± 12; non-BA: 6.4 ± 4.1; p < 0.001) and CD4+FOXP3+ Tregs (BA: 8.5 ± 4.7; non-BA: 2.1 ± 1.9; p < 0.01) infiltrating the portal tract were significantly higher in the BA group. However, the percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) was similar in both groups (BA: 0.32 ± 0.10; non-BA: 0.30 ± 0.13; p = 0.78) (Fig. 2c). These results indicate that Treg infiltration into the portal tract was high, although the proportion of Tregs in CD4+ T cells was consistent in the portal tract of BA liver tissue.
a CD4 and FOXP3-positive cells in the portal tract of BA and non-BA liver. Arrows indicate the CD4+ cells, and arrowheads indicate the CD4+FOXP3+ cells. Scale bar = 20 μm b The mean number of CD4+ cells (non-BA: 6.4 ± 4.1; BA: 26 ± 12; ***p < 0.001) and CD4+FOXP3+ cells (non-BA: 2.1 ± 1.9; BA: 8.5 ± 4.7; ** p < 0.01) infiltrating the portal tract c The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) (non-BA: 0.30 ± 0.13; BA: 0.32 ± 0.10; p = 0.78)
Correlation between clinical data and frequency of infiltrating cells to the portal tract
Next, we examined whether CD4+ T cells and Treg infiltration were associated with the progression of liver injury, liver fibrosis, and prognosis of BA. First, we examined the correlation between each laboratory data (ALT, γ-GTP, T-Bil, D-Bil) and the number of lymphocytes, CD4+ cells, and CD4+FOXP3+ cells, and the percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells). Among them, no correlation was found with ALT, T-bil, or D-bil, but γ-GTP was correlated with the number of CD4+ cells, CD4+FOXP3+ cells, and CD4+FOXP3+ Tregs/CD4+ cells (Table 2). For liver fibrosis, there appeared to be a trend towards increased infiltration of each lymphocyte in livers with moderate (II) fibrosis compared to mild (I) fibrosis, although there was no statistically significant difference in each lymphocyte. In contrast, the percentage of Tregs was similar between livers with mild (I) and moderate (II) fibrosis (Online Resource 1). Lymphocyte infiltration into the portal tract may be associated with the degree of biliary tract damage.
We also investigated the association between these infiltrating cells and the jaundice clearance and outcome at 3 years of BA patients. For jaundice clearance, although we could not test for statistical significance due to the small sample size, there appeared to be no clear associations between the BA and non-BA groups (Online Resource 2). For outcome at 3 years, there was no significant difference between native liver (n = 5) and transplanted (n = 3) in each lymphocyte and the percentage of Tregs (Online Resource 3).
Discussion
Immune dysfunction is a leading hypothesis in the pathogenesis of BA. Tregs are key players in immune homeostasis and are impaired in autoimmune diseases [24,25,26]. Failure of Treg function may be caused by a lack of Tregs with the specificity required to suppress inflammation in an organ, cell-intrinsic defects, or extrinsic factors that impede Treg function, such as resistant effector cells, altered antigen-presenting cell function, or increased proinflammatory cytokines [27].
In this experiment, there was no decrease in the number of infiltrating Tregs. While the absolute number of infiltrated lymphocytes and Tregs into the portal tract increased in BA patients, a consistent proportion of Tregs in CD4+ T cells was observed compared to the non-BA group. Furthermore, the number of these cells was correlated with γ-GTP and appeared to increase with liver fibrosis, although not significantly, probably due to insufficient sample size. Lymphocyte infiltration is a major feature of many forms of chronic liver disease [28]. Our results are very similar to those of a previous study by Ward et al. [29]. In their research, they counted CD4+ cells and FOXP3+ cells infiltrating the portal tract with immunohistochemistry using liver biopsy specimens from chronic hepatitis C patients. They showed a striking number of infiltrating FOXP3+ cells present in the portal tract. A remarkably consistent ratio of total CD4+:FOXP3+ cells in the liver across a wide range of disease states was also observed. Moreover, the average number of CD4+ cells and FOXP3+ cells was found to correlate with the portal inflammation score. These results suggest that Tregs in BA patients are properly recruited to local inflammatory sites due to biliary tract injury.
Our results are discrepant from those of Yang et al. They reported that the number of Foxp3+ cells were significantly higher in BA livers than in healthy controls, but the ratio of Foxp3+T cells to CD4+T cells was significantly lower [18]. Although their results agree with ours in that the absolute number of Tregs is increased in the portal tract, the decrease in the Foxp3+T / CD4+T ratio was not reproducible in our study. Some differences in their experimental settings from ours may have caused this discrepancy. First, they obtained the liver tissues from the hepatic side of the hilar fibrous mass and liver parenchyma from the quadrate lobe, whereas we used tissues from wedge resection of the liver parenchyma. The hilar fibrous mass may have more intense inflammation than the intrahepatic bile ducts we evaluated. Moreover, the hilar fibrous mass may have additional inflammation caused by the surgical invasion, which would bias infiltrating cells toward the effector cells.
Secondly, they selected patients without cholestasis as their controls, such as those with intestinal atresia, whereas we selected patients with cholestasis as often as possible to minimize the potential bias that the cholestasis itself could cause. Hill et al. also used cholestatic control–proven α-1 antitrypsin deficiency and Alagille syndrome, and did not show any difference in infiltrating hepatic Treg numbers between BA and pathological control [30]. It is known that bile acid metabolites promote the generation and differentiation of Treg cells [20, 21], that bile duct ligation results in an expansion of liver Tregs [22], and that the number of CD4+ cells and FOXP3+ cells correlate with the portal inflammation score [29]. In addition, our results suggested that lymphocyte infiltration into the portal tract was correlated with biliary damage and fibrosis. As such, any cholestasis or biliary damage may influence the results of the control group. We believe that a comparison with an appropriate cohort of patients with cholestatic status is better than normal control. The comparison among the three groups of BA, cholestatic control, and healthy control might be more ideal, but the collection of normal control samples has practical and ethical difficulties.
The third difference is that Yang and colleagues evaluated CD4 and Foxp3 in different sections, whereas we used double staining techniques. Although Treg defects are frequently reported in autoimmune diseases, there are frequent discrepancies in the literature. It is known that variation in the choice of study participants and the techniques used to study Treg cells can lead to inconsistency in the results [12].
Our results suggest that Tregs appear to be unsuccessful in suppressing the progressive inflammation of the bile ducts in BA patients, even though they are properly recruited to local sites. Yang et al. demonstrated decreased peripheral Treg cell population and increased interlobular bile duct Foxp3 expressions and speculated that Treg cells are actively recruited to the liver to suppress proinflammatory immune responses and accumulate around the damaged intralobular bile ducts. However, they also showed a decrease in the Foxp3+T cell/Th17 ratio in the portal tracts of BA subjects and presented the possibility that Treg cells may not retain their suppressive functions [18]. Hill et al. also showed unchanged Treg cells and increased Th-17 cells in the portal tract of BA patients compared to cholestatic control patients [30]. Treg/Th17 imbalance has been reported to be important in various chronic inflammatory diseases [31, 32]. As BA is a chronic progressive disease that leads to liver failure, these observations provide interesting insights from a pathological perspective.
Functional impairment of locally accumulated Tregs is thus a topic to be addressed in the future. We previously examined the function of the circulating Tregs of BA patients using a suppression assay, the results of which were similar to those of the control group [33]. If Tregs themselves are originally dysfunctional, genetically for example, dysfunction should be observed in circulating Tregs. If the Treg dysfunction is limited to the local area, the local environment must be causing Treg dysfunction either directly or through altered resistance of effector T cells to Tregs.
Recently, there have been conflicting reports on whether Treg frequency and/or function is actually reduced in autoimmune diseases [27]. Compelling evidence suggests that conventional T cells (Tcon cells) refractory to Treg suppression also act as mediators of autoimmune disease [34]. Tcon cells can become insensitive to Treg-mediated suppression when intracellular signaling pathways have been modified by mutations or through extracellular signals such as strong activation or a specific cytokine milieu that induces Tcon cell–intrinsic changes [35]. The latter mechanism refers to potentially pathogenic Tcon cells that have become resistant to Treg suppression, a phenomenon observed in several autoimmune diseases [34]. Extracellular factors include IL-6, TNFα, and IL-1β, and intracellular signaling molecules linked to Tcon resistance include Cbl-b, TRAF6, and SHP-1 [34]. These factors are thought to finally act on phosphatidylinositol-3 kinase (PI3K)/Akt signaling, which may be at the heart of Tcon resistance [34]. Yang et al. investigated the cytokine environment within the liver of BA and HC patients and found that the expression of cytokines IL-1β and IL-6 was enhanced in BA livers. They pointed out the possibility that in a cytokine microenvironment rich in IL-1β and IL-6, these Treg cells may not retain their suppressive functions, which may contribute to the disease [18]. Conversely, recent reports of single-cell RNA profiling using BA liver tissues have not shown elevated levels of IL-1β, IL-6, and TNFα. The cytokine microenvironments and Tcon resistance in BA liver remain unclear and are to be addressed in further studies.
There are several limitations in the present study. First, the sample size was small to evaluate the association of liver fibrosis and jaundice clearance with Tregs. Second, designing optimal inclusion criteria for the non-BA group was challenging. We employed patients with cholestatic disorders other than BA for the control group, but the differences in cholestatic parameters between the BA and non-BA groups reached statistical significance. Furthermore, since the non-BA group contained various primary diseases, the possibility that Treg frequency is affected by the primary disease cannot be excluded.
Conclusion
We investigated the infiltrating regulatory T-cells in the portal tract of biliary atresia and their correlation to clinical parameters, including laboratory data associated with cholestasis, liver fibrosis, and jaundice clearance. The absolute number of Treg infiltration into the portal tract was increased in BA patients, whereas the Treg proportion in CD4+ cells was consistent, suggesting that Tregs are unsuccessful at suppressing the progressive inflammation of the bile ducts in BA patients, even though they are adequately recruited to local sites. Investigation of the suppressive function of Tregs in the local environment, together with extrinsic factors such as Treg resistance, is warranted.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bezerra JA, Wells RG, Mack CL et al (2018) Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology 68:1163–1173. https://doi.org/10.1002/hep.29905
Chardot C (2006) Biliary atresia. Orphanet J Rare Dis 1:28. https://doi.org/10.1186/1750-1172-1-28
Asai A, Miethke A, Bezerra JA (2015) Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 12:342–352. https://doi.org/10.1038/nrgastro.2015.74
Nizery L, Chardot C, Sissaoui S et al (2016) Biliary atresia: clinical advances and perspectives. Clin Res Hepatol Gastroenterol 40:281–287. https://doi.org/10.1016/j.clinre.2015.11.010
Nio M (2017) Japanese biliary atresia registry. Pediatr Surg Int 33:1319–1325. https://doi.org/10.1007/s00383-017-4160-x
Mack CL, Feldman AG, Sokol RJ (2012) Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis 32:307–316. https://doi.org/10.1055/s-0032-1329899
Sokol RJ, Mack C, Narkewicz MR, Karrer FM (2003) Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr 37:4–21. https://doi.org/10.1097/00005176-200307000-00003
Hartley JL, Davenport M, Kelly DA (2009) Biliary atresia. Lancet 374:1704–1713. https://doi.org/10.1016/S0140-6736(09)60946-6
Wang J, Xu Y, Chen Z et al (2020) Liver immune profiling reveals pathogenesis and therapeutics for biliary atresia. Cell 183:1867-1883.e26. https://doi.org/10.1016/j.cell.2020.10.048
Lakshminarayanan B, Davenport M (2016) Biliary atresia: a comprehensive review. J Autoimmun 73:1–9. https://doi.org/10.1016/j.jaut.2016.06.005
Wing JB, Sakaguchi S (2012) Multiple treg suppressive modules and their adaptability. Front Immunol 3:178. https://doi.org/10.3389/fimmu.2012.00178
Grant CR, Liberal R, Mieli-Vergani G et al (2015) Regulatory T-cells in autoimmune diseases: challenges, controversies and—yet—unanswered questions. Autoimmun Rev 14:105–116. https://doi.org/10.1016/j.autrev.2014.10.012
Sakaguchi S, Mikami N, Wing JB et al (2020) Regulatory T cells and human disease. Annu Rev Immunol 38:541–566. https://doi.org/10.1146/annurev-immunol-042718-041717
Miethke AG, Saxena V, Shivakumar P et al (2010) Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol 52:718–726. https://doi.org/10.1016/j.jhep.2009.12.027
Lages CS, Simmons J, Chougnet CA, Miethke AG (2012) Regulatory T cells control the CD8 adaptive immune response at the time of ductal obstruction in experimental biliary atresia. Hepatology 56:219–227. https://doi.org/10.1002/hep.25662
Tucker RM, Feldman AG, Fenner EK, Mack CL (2013) Regulatory T cells inhibit Th1 cell-mediated bile duct injury in murine biliary atresia. J Hepatol 59:790–796. https://doi.org/10.1016/j.jhep.2013.05.010
Li K, Zhang X, Yang L et al (2016) Foxp3 promoter methylation impairs suppressive function of regulatory T cells in biliary atresia. Am J Physiol Gastrointest Liver Physiol 311:G989–G997. https://doi.org/10.1152/ajpgi.00032.2016
Yang Y, Liu Y-J, Tang S-T et al (2013) Elevated Th17 cells accompanied by decreased regulatory T cells and cytokine environment in infants with biliary atresia. Pediatr Surg Int 29:1249–1260. https://doi.org/10.1007/s00383-013-3421-6
Saito T, Sakamoto A, Hatano M et al (2017) Systemic and local cytokine profile in biliary atresia. Eur J Pediatr Surg 27:280–287. https://doi.org/10.1055/s-0036-1592136
Hang S, Paik D, Yao L et al (2019) Bile acid metabolites control TH17 and treg cell differentiation. Nature 576:143–148. https://doi.org/10.1038/s41586-019-1785-z
Campbell C, McKenney PT, Konstantinovsky D et al (2020) Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581:475–479. https://doi.org/10.1038/s41586-020-2193-0
Katz SC, Ryan K, Ahmed N et al (2011) Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol 187:1150–1156. https://doi.org/10.4049/jimmunol.1004077
Weerasooriya VS, White FV, Shepherd RW (2004) Hepatic fibrosis and survival in biliary atresia. J Pediatr 144:123–125
Longhi MS, Mieli-Vergani G, Vergani D (2021) Regulatory T cells in autoimmune hepatitis: an updated overview. J Autoimmun 119:102619. https://doi.org/10.1016/j.jaut.2021.102619
Miyara M, Gorochov G, Ehrenstein M et al (2011) Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev 10:744–755. https://doi.org/10.1016/j.autrev.2011.05.004
Dominguez-Villar M, Hafler DA (2018) Regulatory T cells in autoimmune disease. Nat Immunol 19:665–673. https://doi.org/10.1038/s41590-018-0120-4
Long SA, Buckner JH (2011) CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol 187:2061–2066. https://doi.org/10.4049/jimmunol.1003224
Friedman SL (2004) Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol 1:98–105. https://doi.org/10.1038/ncpgasthep0055
Ward SM, Fox BC, Brown PJ et al (2007) Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol 47:316–324. https://doi.org/10.1016/j.jhep.2007.03.023
Hill R, Quaglia A, Hussain M et al (2015) Th-17 cells infiltrate the liver in human biliary atresia and are related to surgical outcome. J Pediatr Surg 50:1297–1303. https://doi.org/10.1016/j.jpedsurg.2015.02.005
Zhang S, Gang X, Yang S et al (2021) The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol 12:678355. https://doi.org/10.3389/fimmu.2021.678355
Lourenço JD, Ito JT, de Martins MA et al (2021) Th17/Treg imbalance in chronic obstructive pulmonary disease: clinical and experimental evidence. Front Immunol 12:804919. https://doi.org/10.3389/fimmu.2021.804919
Oita S, Saito T, Sakamoto A et al (2022) Frequency and function of circulating regulatory T-cells in biliary atresia. Pediatr Surg Int 39:23. https://doi.org/10.1007/s00383-022-05307-8
Mercadante ER, Lorenz UM (2016) Breaking free of control: how conventional T cells overcome regulatory T cell suppression. Front Immunol 7:193. https://doi.org/10.3389/fimmu.2016.00193
Buckner JH (2010) Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol 10:849–859. https://doi.org/10.1038/nri2889
Acknowledgements
The authors thank Ms. Takana Motoyoshi (K. I. Stainer, Inc.) for their technical assistance.
Funding
This work was supported by JSPS KAKENHI (Grant Numbers 15K10917 and 18K08535). None of the authors have any relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Conceptualization: SO, TS; Methodology: SO, RH, J-II; Formal analysis and investigation: SO, RH, TF, YK; Writing—original draft preparation: SO; Writing—review and editing: KT, SK, AT, TH; Funding acquisition: TS; Supervision: J-II, TH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the Ethics Committee of the Graduate School of Medicine, Chiba University (approval number 908) and the Institutional Review Board of Chiba Children’s Hospital (2017–046).
Consent to participate and publish
Informed consent was obtained from all participants (or their parents) in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
383_2023_5547_MOESM1_ESM.tiff
Online Resource 1: Correlation between fibrosis score and frequency of infiltrating cells to the portal tract (a) The number of lymphocytes/10,000 μm2 [mild (I): 26 ± 12; moderate (II): 34 ± 14; p = 0.22] (b) The mean number of CD4+ cells [mild (I): 11 ± 8.8; moderate (II): 2 4 ± 15; p = 0.05] (c) The mean number of CD4+FOXP3+ cells [mild (I): 3.2 ± 2.7; moderate (II): 8.0 ± 5.7; p = 0.05] (d) The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) [mild (I): 0.30 ± 0.12; moderate (II): 0.32 ± 0.11; p = 0.66]. Supplementary file1 (TIFF 2209 KB)
383_2023_5547_MOESM2_ESM.tiff
Online Resource 2: Correlation between jaundice clearance and frequency of infiltrating cells to the portal tract (a) The number of lymphocytes/10,000μm2 (failed jaundice clearance: 30 ± 18; achieved jaundice clearance: 38 ± 11) (b) The mean number of CD4+ cells (failed: 31 ± 18; achieved: 25 ± 11) (c) The mean number of CD4+FOXP3+ cells (failed: 12±7.6; achieved: 7.4±3.7) (d) The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) (failed: 0.37 ± 0.033; achieved: 0.30 ± 0.11). Supplementary file2 (TIFF 2313 KB)
383_2023_5547_MOESM3_ESM.tiff
Online Resource 3: Correlation between outcome at 3 years and frequency of infiltrating cells to the portal tract (a) The number of lymphocytes/10,000 μm2 [native liver: 35 ± 10; transplanted: 37 ± 18; p = 0.87] (b) The mean number of CD4+ cells [native liver: 21 ± 3.7; transplanted: 36 ± 16; p = 0.07] (c) The mean number of CD4+FOXP3+ cells [native liver: 6.2 ± 2.4; transplanted: 12 ± 5.5; p = 0.07] (d) The percentage of Tregs among CD4+ cells (CD4+FOXP3+ Tregs/CD4+ cells) [native liver: 0.30 ± 0.13; transplanted: 0.34 ± 0.051; p = 0.61]. Supplementary file3 (TIFF 2443 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oita, S., Saito, T., Hashimoto, R. et al. Frequency of infiltrating regulatory T-cells in the portal tract of biliary atresia. Pediatr Surg Int 39, 259 (2023). https://doi.org/10.1007/s00383-023-05547-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05547-2