Abstract
Purpose
Preterm infants are more susceptible to necrotizing enterocolitis (NEC) than term Queryinfants. This may be due to a relative paucity of Lgr5+ or Bmi1+-expressing intestinal stem cells (ISCs) which are responsible for promoting intestinal recovery after injury. We hypothesized that the cellular markers of Lgr5+ and Bmi1+, which represent the two distinct ISC populations, would be lower in younger mice compared to older mice. In addition, we hypothesized that experimental NEC would result in a greater loss of Lgr5+ expression compared to Bmi1+ expression.
Methods
Transgenic mice with EGFP-labeled Lgr5 underwent euthanasia at 10 different time points from E15 to P56 (n = 8–11/group). Lgr5+-expressing ISCs were quantified by GFP ELISA and Bmi1+ was assessed by qPCR. In addition, Lgr5EGFP mice underwent experimental NEC via formula feeding and hypoxic and hypothermic stress. Additional portions of the intestine underwent immunostaining with anti-GFP or anti-Bmi1+ antibodies to confirm ELISA and PCR results. For statistical analysis, p < 0.05 was significant.
Results
Lgr5+ and Bmi1+expression was lowest in embryonal and early postnatal mice and increased with age in all segments of the intestine. Experimental NEC was associated with loss of Lgr5+-expressing ISCs but no significant change in Bmi1+ expression.
Conclusion
Lgr5+ and Bmi1+ expression increase with age. Lgr5+-expressing ISCs are lower following experimental necrotizing enterocolitis while Bmi1+ expression remains relatively unchanged. Developing a targeted medical therapy to protect the low population of ISCs in preterm infants may promote tissue recovery and regeneration after injury from NEC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Necrotizing enterocolitis (NEC) is a devastating disease that affects the gastrointestinal tract of preterm infants [1]. Despite decades of research, there have been only limited advancements in understanding the complex pathophysiology associated with NEC, and even more limited advancements in the development of targeted therapies [2]. NEC often evolves rapidly and presents as abdominal distention, feeding intolerance, and sepsis [3]. Mortality is estimated at 20% in all infants with NEC, 40% for infants who require surgery, and 50% for extremely low birthweight infants who require surgery [4].
To improve outcomes in NEC and develop a targeted medical therapy, it is important to better define the pathophysiology. It is also critical to better understand why preterm infants are more susceptible to NEC than their term counterparts. Premature infants are at high risk for the development of NEC in part due to perturbations of intestinal peristalsis [5, 6], decreased intestinal mucous production [7], leaky epithelium that allows bacteria to translocate [8], and immaturity of enzymes for digesting nutrients [9]. However, a diminished capacity for intestinal repair following injury is also highly likely, and this may be due to a relative paucity of intestinal stem cells (ISCs) [10, 11].
Intestinal stem cells play a critical role during injury, where they repair damaged tissue and repopulate the intestine with functional cells to facilitate recovery [12, 13]. This process is known as intestinal restitution. NEC often causes severe irreversible injury to the intestine of preterm infants, and this may be due to younger infants having a smaller reservoir of ISCs to replace injured epithelial cells in the gut.
There are two distinct intestinal stem cell populations that reside in the intestinal crypts. Bmi1+ (B lymphoma Mo-MLV insertion region 1 homolog) expressing cells reside at the + 4 position in the crypts of the intestine, are relatively quiescent and are capable of differentiating into all cell types of the intestine [14]. In contrast to Bmi1+ cells, the Lgr5 (leucine-rich repeat-containing G-protein coupled receptor 5) surface protein expressing ISC resides deep in the crypts of the intestinal villi and is mitotically quite active [15]. There are an estimated 4–6 Lgr5+ ISCs per crypt [16]. These intestinal stem cells divide frequently to rapidly renew the intestinal epithelium and are also progenitors to all cell types of the intestinal villi, including enterocytes, goblet cells, enteroendocrine cells, and Paneth cells [17].
It has been suggested that Bmi1+ and Lgr5+ mark an overlapping or redundant stem cell system. Transgenic mice with fluorescent-labeled Lgr5+ or Bmi1+ cells were studied in quiescent homeostatic states. Lgr5+ cells cycled more rapidly and were more efficient at generating progeny compared to Bmi1+. Lgr5+ cells also responded more robustly to Wnt agonists and antagonists, thereby shedding light on stimuli for rapid cell division. However, when the bowel was injured with radiation, the Lgr5+ population was eradicated quickly while the Bmi1+ population survived and expanded. Bmi1+ cells gave rise to additional Lgr5 + cells. These data suggest that the Lgr5 + population is rapidly cycling and may be more involved in homeostasis, while Bmi1 + cells are more injury resistant and help to repopulate the gut after severe injury [18].
The objective of this study was to quantify the number of Lgr5+ and Bmi1+-expressing ISCs and assess how age affects the relative abundance of these cell populations. We hypothesized that increasing age would directly correlate with the abundance of ISC markers in the duodenum, terminal ileum, and colon. In addition, we hypothesized that experimental NEC would be associated with a greater loss of Lgr5+ expression in the terminal ileum compared to Bmi1 +.
Methods
Tissue procurement and genotyping
All research followed ethical guidelines under an IACUC-approved protocol at the Indiana University School of Medicine. To identify the proportion of Lgr5+-expressing ISCs, we used a mutant mouse with an enhanced green fluorescent protein (EGFP) knocked into the Lgr5 gene locus (Genentech, San Francisco, CA). Detection of EGFP in heterozygous Lgr5EGFP mice served as a marker of Lgr5+-expressing ISCs, as described by Tian et al. [19]. Tissue was obtained from tail cuts for genotyping. DNA was extracted with 50 mM sodium hydroxide at 95 ºC for 1 h. DNA was amplified with Taq polymerase (Cat # M712B, Thermo Fisher Scientific, Waltham, MA, USA) and primers as shown in Appendix 1 . Only heterozygous Lgr5EGFP mice were used for analysis as a homozygous Lgr5EGFP mutation is lethal (Fig. 1).
Heterozygous Lgr5EGFP male mice were bred with female C57BL/6 J mice (Jackson Labs, Bar Harbor, ME). Timed breeding was utilized to measure the precise age of the progeny. Mice were euthanized at 2 embryonal (E) ages and 8 postnatal (P) ages: E15, E18, P1, P3, P5, P7, P14, P21, P28, and P56. For embryo tissue procurement, pregnant dams underwent carbon dioxide (CO2) euthanasia. A midline laparotomy was performed, and embryos were removed from the uterine horns. Intestine was isolated and identified using a dissecting microscope. For E15 embryos, the gut was explanted and separated into 2 segments: ileum and colon. For all other age groups, the gut was mature enough to separate into 3 segments: duodenum, terminal ileum, and ascending colon. Pups remained with dams and breastfed until 21 days at which time they were weaned. Pups from age P1 to P7 were euthanized via cervical decapitation, and mice from P14 to P56 underwent CO2 euthanasia.
Lgr5+ stem cell quantification by ELISA
Segments of the intestine were harvested immediately after euthanasia and were snap frozen in liquid nitrogen and stored at –80 ºC. Tissue was homogenized in a 4 ºC cold room. The intestine was placed into 1.5 mL Eppendorf tubes (Cat # 05,408,129, Thermo Fisher Scientific) with 1 scoop of 0.9–2.0 mm stainless steel beads, and 5 individual 3.2 mm stainless steel beads (Cat # SSB14B and SSB32, MIDSCI, Valley Park, MO, USA). In addition, 200 µl of RIPA buffer (Cat # 89,900, Thermo Fisher Scientific) with both 1:100 dilution protease inhibitor and phosphatase inhibitor (Cat # P8340 and P5726, Sigma-Aldrich Company, St. Louis, MO, USA) was added. The tissue was homogenized with a bullet blender (Cat # BBX24B, Next Advance, Troy, NY, USA) for 6 min. After homogenization, lysates were centrifuged at 12,000 RPM for 5 min and supernatants were stored at –80 ºC. Total protein was quantified by Bradford Assay using a SpectraMax M2e Multi-Mode microplate reader spectrophotometer (Molecular Devices, San Jose, CA, USA). Protein input was equivalent across all groups and EGFP expression was measured via ELISA (Cat # Ab171581, Abcam, Cambridge, UK). Assays were performed in duplicate per the manufacturer’s instructions, and GFP ELISA was repeated to verify results (n = 8–11 mice per group).
Bmi1+ stem cell quantification by real-time PCR
Tissue was disrupted with a Fisherbrand Pellet Pestle Cordless Motor (Fisher Scientific) in Trizol (Life Technologies/Thermo Fisher). RNA was isolated using Trizol and quantitated using a Nanodrop 2000 spectrophotometer (Thermo Fisher). cDNA was synthesized from 273.5 ng of RNA using ABScript II cDNA First-Strand Synthesis Kit (ABclonal). Real-time PCR was performed using the Taq Man gene expression system (Thermo Fisher), with Bmi1 probe (FAM) (ref seq NM_007552.4) and GAPDH probe (VIC) (ref seq NM_001289726.1, NM_008084.3) as an internal control (Appendix 1). Amplification was performed using QuantStudio 6 Real-Time PCR System (Applied Biosystems/Thermo Fisher). Real-time PCR program was as follows: hold stage of 50 °C for 2 min, 95 °C for 10 min, followed by a 40 cycle PCR stage of 95 °C for 15 s, 60 °C for 1 min. Values for Bmi1 and GAPDH were generated and Δct was calculated as 2(GAPDH−BMI1).
Experimental NEC model
Experimental NEC was based on the model described by Zani et al. [20, 21]. Heterozygous Lgr5EGFP male mice were bred with female C57BL/6 J mice. Two groups of pups were studied: (1) breastfed controls (n = 14) and (2) NEC (n = 19). Experimental pups underwent permanent maternal separation on P5 and were taken to satellite housing in a temperature-controlled incubator. Control pups were left with their mothers to breastfeed. Pups received a total of 300 kcal/kg/day of Esbilac milk replacer (PetAg, Hampshire, IL, USA) fortified with Similac Advance powder (Similac, Columbus, OH, USA) via gavage feeding with a 1.9 French catheter. Feeds were also supplemented with 8 mg/kg lipopolysaccharide (LPS) isolated from Escherichia Coli O111:B4 (Cat # L4391, Sigma-Aldrich Company). Pups received stress via 5% hypoxia for 10 min three times daily, and 4 ºC for 12 min hypothermia twice daily. Pups were euthanized via cervical decapitation on P9. Tail cuts were saved for post-mortem genotyping.
Immunohistochemistry
Breastfed control and NEC segments of terminal ileum were harvested immediately after euthanasia and were fixed with paraformaldehyde for 36 h at 4 ºC and dehydrated in 70% ethanol for 24 h. After dehydration, tissues were paraffin embedded and sectioned with a microtome. Specimens were subsequently stained with a rabbit anti-GFP antibody at 1:1000 dilution (Cat # Ab6556, Abcam), as done by Todaro et al. to measure stem cell quantity [22]. The intensity of anti-GFP staining was analyzed at intestinal crypts and acted as a marker of Lgr5+-expressing ISCs. Additional segments were stained with a rabbit anti-Bmi1+ antibody at 1:200 dilution (Cat # 254,253). Staining was quantified using the IHC Profiler plugin on ImageJ [23].
Statistical analyses
Continuous data were reported as the mean and standard error of the mean (SEM). ELISA and PCR data were tested for normality and were compared using one-way ANOVA with Tukey’s multiple comparisons test. Experimental NEC survival data were compared using a Log-Rank (Mantel–Cox) test, while all control and experimental NEC data were compared using the Mann–Whitney U test. All statistical analyses were performed, and figures were created using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). P values less than 0.05 were considered significant.
Results
Duodenum
The proportion of Lgr5+ expression in the duodenum was lowest at E18 (288.1 ng GFP/g protein ± 19.4, Fig. 1A). After weaning, they continued to rise with age and peaked at P56 (2972.0 ng GFP/g protein ± 316.0, p < 0.0001 vs. E18). When compared to P21 (weaning age), Lgr5+-expressing ISCs were lower at days E18, 1, 3, 4, and 7 (Fig. 2A). The proportion of Bmi1+ expression also increased with age. When compared to E18, Bmi1+ expression was higher at day of life 7 (p < 0.001), 21 (p < 0.05), and 28 (p < 0.05, Fig. 1B).
Terminal ileum
Lgr5+ expression was lowest at the embryonic stages (E15 = 395.8 ng GFP/g protein ± 43.2, E18 = 392.9 ng GFP/g protein ± 29.9, Fig. 3A). As the pups continued to age, intestinal stem cell Lgr5+ peaked at P21 (5564.0 ng GFP/g protein ± 727.0, p < 0.0001 vs. E15). After weaning at P21 (5564 ng GFP/g protein ± 727), the proportion of Lgr5 expression decreased and continued to drop until the oldest age point assessed (3770.0 ng GFP/g protein ± 473.2, p < 0.05, Fig. 3A). The proportion of Bmi1+ expression also increased with age in the terminal ileum. When compared to E18, Bmi1+ expression was higher at all other assessed timepoints (p < 0.05, Fig. 3B).
Colon
In the ascending colon, Lgr5+ expression was lowest at the embryonic stages (E15 = 105.6 ng GFP/g protein ± 11.6, E18 = 237.4 ng GFP/g protein ± 22.1, Fig. 4A). After birth, Lgr5+ expression increased significantly and peaked at P7 (1767.0 ng GFP/g protein ± 193.1, p < 0.0001 vs. E15) and remained elevated with breastmilk exposure. After weaning at P21, the Lgr5+-expressing ISC population was still higher than in embryonic stages, but was not statistically different from P21 (Fig. 4A). The proportion of Bmi1+ expression also increased with age in the colon. When compared to E15, Bmi1+ expression was higher at the day of life 14, 21, 28, and 56 (p < 0.05). When compared to P21, days E15, E18, 3, and 7 were significantly lower (p < 0.05). Bmi1+ expression in the days following weaning was not significantly different than P21 (Fig. 4B).
Experimental NEC
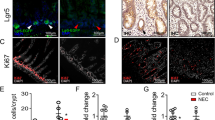
Overall mortality from experimental NEC was 26.3% (p = 0.04 vs. breastfed control, Fig. 5). After undergoing experimental NEC, pups had a decreased expression of Lgr5 in the terminal ileum when compared to breastfed controls (NEC = 1174 ng GFP/g protein ± 188.3, breastfed control = 2162.0 ± 259.1 ng GFP/g protein, p = 0.0023, Fig. 6A). Analysis of Bmi1+ expression by PCR noted a higher mean value in the NEC group, but this was not statistically different from breastfed controls (p = 0.53, Fig. 6B). When comparing the change in immunostaining between breastfed controls and those with NEC (Breastfed control minus NEC), there was a higher loss of Lgr5+ staining (Fig. 7A, B-Delta Lgr5) compared to Bmi1+ staining (Fig. 7C, D-Delta Bmi1) (p = 0.004).
Lgr5+ loss is more prominent in NEC. Representative immunohistochemistry-stained images of A Lgr5+ breastfed control and B NEC, as well as Bmi1+ C breastfed control and D NEC. The loss of Lgr5+ staining as measured by Image J between breastfed and NEC pups (Delta Lgr5) was significantly more pronounced than Bmi1+ staining in the same population (Delta Bmi1). * = p < 0.05 vs. Delta Lgr5. Arrow represents positive staining
Discussion
A plethora of endocrine, paracrine, and transcription factors orchestrate intestinal morphogenesis from the embryonic stage through adulthood. The involvement of factors regulating proliferation and differentiation during the neonatal period declines over time during maturation to adulthood, suggesting that adult stem cells have unique requirements of factors and cofactors for intestinal stem cell maintenance and function distinct from what roles were predominant during the early neonatal period [24].
The control of intestinal stem cell proliferation, differentiation, and self-renewal in the postnatal period is regulated by multiple developmental pathways, which are likely evolutionarily conserved. These include Hedgehog, BMP, Wnt, and Notch signaling cascades. These pathways work to help form the intestinal crypts, separate the cells into different cell types, and promote the development of the intestinal villus. For example, Sox9, a transcription factor located downstream of Wnt, is expressed throughout the epithelium as early as embryonic day E13.5 but becomes restricted to the crypts in the adult intestines. Sox9 represses Cdx2 and Muc2, which are two genes that assist in intestinal differentiation. Other factors that likely regulate ISC development include various hormones, such as thyroid hormone, which significantly increases immediately after birth. Elevations in thyroid hormone coincide with alterations in Blimp1, which is strongly expressed throughout the intestine in fetal stages but becomes restricted to the intervillous regions in neonates [24].
The pathophysiology of necrotizing enterocolitis is multifactorial, complex, and remains poorly understood [25]. It is critical to better understand the disease process to discern why preterm infants are heavily affected by this devastating disease. In our study, we observed that the Lgr5+ and Bmi1+expression was lowest in embryonal mice in all segments of the intestine that were studied. These markers, which represent the ISC populations, appeared to increase with age until peaking sometime in the first month of life. These low cell numbers in the embryonal stage and early postnatal period may prevent infants from mounting an appropriate response to injury, thereby making them more susceptible to the effects of NEC.
The quantity of Lgr5 expression was most interesting in the terminal ileum, where it remained in lower quantity for an extended period of time (E15–P7) and then underwent a sharp increase at P21. Lgr5 expression became less populous immediately after weaning and continued to decline as the mice aged. These data may help to shed light on additional mechanisms of terminal ileal susceptibility, which may include fewer ISCs to repopulate the gut in this region after injury.
While there is currently no targeted medical therapy for NEC, breastmilk is known to reduce the incidence of NEC [26,27,28]. There are currently different theories on the mechanism of how breastmilk prevents infants from developing NEC, but it may act by altering the microbiome or increasing mesenteric perfusion [29,30,31]. Our data demonstrated an association between higher levels of Lgr5 expression within the terminal ileum with exposure to breastmilk and a decrease in this expression following weaning. Therefore, as suggested by Chen et al., an additional protective property of breastmilk may be to increase the population of ISCs [32]. Further studies are certainly needed.
Although the provision of breastmilk to preterm infants is one of the most widely studied preventative strategies for NEC, other therapies may be as equally important. Probiotics have been shown in numerous studies to protect infants from necrotizing enterocolitis [33,34,35]. Furthermore, modulation of the intestinal microbiome also appears to have effects on the intestinal stem cell niche [36, 37]. Therefore, it is possible that probiotics administered to preterm infants may exhibit their protective effects, in part, by altering the native microbiome and modulating intestinal stem cells. Additional therapies that have shown protective effects to intestinal stem cells (in animal models) include heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cell therapy [32, 38].
Lastly, we noted an association between a significant loss of Lgr5+ expression in the terminal ileum for mice undergoing experimental NEC. The terminal ileum is traditionally at-risk during NEC, possibly due to lower blood flow in this area [39,40,41]. Our data contribute to these findings by suggesting that lower numbers of ISCs in the early postnatal period may make neonates more susceptible to NEC given that they already had a relatively low number of ISCs compared to their term counterparts. Therefore, it is possible that a correlation exists between the number of ISCs and the ability of preterm neonates to repopulate the gut with functional cells after injury. Our data did not demonstrate that experimental NEC resulted in a significant decrease in Bmi1 + expression, suggesting that Lgr5+-expressing ISCs may be more heavily affected during NEC. This finding is also consistent with previously published data, where Bmi1 + cells were more resistant to ischemic and radiation-induced injury and continued to be expressed in low populations in the terminal ileum following injury [14, 18, 42].
Therefore, our data suggest that intestinal stem cells, as measured by Lgr5+ and Bmi1+ expression within the intestines, are lowest in the embryonal and early postnatal periods and increase with age. The change in Lgr5+ expression between NEC and breastfed control mice was more robust than Bmi1+, suggesting that the Lgr5+ ISC population may be more involved in NEC pathogenesis than the Bmi1+ population.
Limitations
There are several limitations to this study. First, the measurement of Lgr5 and Bmi1+ expression had higher variability in the postnatal groups compared to the embryonal groups. This may have been due to the presence of stool burden in the intestines in the postanal groups. The Bradford assay may have also been measuring proteins present in the stool as well as the bowel, causing more variability in the data when compared to embryonal groups. The intestines were thoroughly flushed with saline prior to being frozen to minimize this effect, and the data may have been less variable for the embryologic ages given that there was no stool in the colon.
Second, we measured markers of ISCs by measuring the expression of Lgr5EGFP mutant surface protein, and Bmi1 + RNA rather than directly measuring Lgr5 and Bmi1. Using a protein or RNA level as a surrogate marker for cell populations may not always be accurate and could even be misleading, as cells may increase in number while simultaneously downregulating a surface protein. Conversely, cells may be injured and decrease in number and upregulate a particular surface protein to compensate. One may argue it is more accurate to measure cell count by measuring Lgr5+ or Bmi1+ expression with flow cytometry, as shown by Nigmatullina et al. [43]. Given that we measured ISC populations in 10 ages of mice in 3 different portions of bowel, flow cytometry would have been challenging and cumbersome. In addition, we did not assess whether the presence of this knock-in mutation affected native Lgr5+ ISCs in the bowel. However, the use of this same heterozygous Lgr5EGFP transgenic mouse to measure Lgr5EGFP as a surrogate marker of the Lgr5+-ISC population has been performed and demonstrated to be accurate [19].
Future directions
Most of our previous work has studied the effectiveness of mesenchymal stem cells (MSCs) for the treatment of intestinal ischemia and necrotizing enterocolitis [44,45,46,47]. The paracrine release of hydrogen sulfide gas is thought to be a major contributor to the effectiveness of these stem cells [45, 48,49,50,51]. MSCs and hydrogen sulfide compounds bring about increased mesenteric perfusion and improved functional outcomes in models of intestinal ischemia and NEC. It is unknown though, how hydrogen sulfide impacts the intestinal stem cell niche. It is possible that hydrogen sulfide works to protect intestinal stem cells so that they can participate in intestinal restitution during injury. Future studies will examine the impact of MSCs and hydrogen sulfide compounds on intestinal stem cells and their ability to facilitate repopulation of the gut following injury.
Conclusion
Markers of the intestinal stem cell population, such as Lgr5 and Bmi1, are lowest in embryonal and early postnatal mice and appear to increase with age. In addition, a loss of Lgr5+ expression was associated with experimental necrotizing enterocolitis, suggesting that the Lgr5+-expressing intestinal stem cell population may be involved in NEC pathogenesis. Conversely, Bmi1 + expression did not significantly decrease in number after experimental NEC, suggesting that this cell population may be relatively resistant to NEC. As the search for a targeted medical treatment for NEC continues, this study demonstrates the importance of developing a therapy to protect the already low population of ISCs in neonatal infants, so they can promote tissue recovery and regeneration after injury.
References
Kovler ML, Sodhi CP, Hackam DJ (2020) Precision-based modeling approaches for necrotizing enterocolitis. Dis Model Mech 13(6):044388. https://doi.org/10.1242/dmm.044388
Hackam DJ, Sodhi CP, Good M (2019) New insights into necrotizing enterocolitis: From laboratory observation to personalized prevention and treatment. J Pediatr Surg 54(3):398–404
Zani A, Pierro A (2015) Necrotizing enterocolitis controversies and challenges. F1000Res 4:F1000
Jones IH, Hall NJ (2020) Contemporary outcomes for infants with necrotizing enterocolitis-a systematic review. J Pediatr 220:86-92 e3
Berseth CL (1989) Gestational evolution of small intestine motility in preterm and term infants. J Pediatr 115(4):646–651
Claud EC (2009) Neonatal necrotizing enterocolitis -inflammation and intestinal immaturity. Anti-inflamm Anti-allergy Agents Med Chem 8(3):248–259
Snyder JD, Walker WA (1987) Structure and function of intestinal mucin: developmental aspects. Int Arch Allergy Appl Immunol 82(3–4):351–356
Rouwet EV et al (2002) Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res 51(1):64–70
Lebenthal A, Lebenthal E (1999) The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr 23(5 Suppl):S3-6
Neal MD et al (2012) Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem 287(44):37296–37308
Li B et al (2019) Impaired Wnt/beta-catenin pathway leads to dysfunction of intestinal regeneration during necrotizing enterocolitis. Cell Death Dis 10(10):743
Bankaitis ED et al (2018) Reserve stem cells in intestinal homeostasis and injury. Gastroenterology 155(5):1348–1361
Metcalfe C et al (2014) Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 14(2):149–159
Sangiorgi E, Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40(7):915–920
Barker N et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165):1003–1007
Bjerknes M, Cheng H (1999) Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116(1):7–14
Munoz J et al (2012) The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J 31(14):3079–3091
Yan KS et al (2012) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A 109(2):466–471
Tian H et al (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478(7368):255–259
Zani A et al (2008) Assessment of a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg 18(6):423–426
Jilling T et al (2006) The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol 177(5):3273–3282
Todaro F et al (2019) Regulation of kit expression in early mouse embryos and es cells. Stem Cells 37(3):332–344
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imagej: 25 years of image analysis. Nat Methods 9(7):671–675
Venkatraman A et al (2021) Intestinal stem cell development in the neonatal gut: pathways regulating development and relevance to necrotizing enterocolitis. Cells 10(2):312
Hackam D, Caplan M (2018) Necrotizing enterocolitis: pathophysiology from a historical context. Semin Pediatr Surg 27(1):11–18
Lucas A, Cole TJ (1990) Breast milk and neonatal necrotising enterocolitis. Lancet 336(8730):1519–1523
Meinzen-Derr J et al (2009) Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol 29(1):57–62
Patel AL, Kim JH (2018) Human milk and necrotizing enterocolitis. Semin Pediatr Surg 27(1):34–38
Sitarik AR et al (2017) Breast milk transforming growth factor beta is associated with neonatal gut microbial composition. J Pediatr Gastroenterol Nutr 65(3):e60–e67
Guner YS et al (2011) P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis. Lab Invest 91(11):1668–1679
Good M et al (2016) The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr 116(7):1175–1187
Chen CL et al (2012) Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest 92(3):331–344
Chang CM et al (2022) Effects of probiotics on gut microbiomes of extremely preterm infants in the neonatal intensive care unit: a prospective cohort study. Nutrients 14(15):3239
Liu H et al (2022) Safety and efficacy of probiotics in the prevention of necrotizing enterocolitis in premature and/or low-birthweight infants: a systematic review and meta-analysis. Transl Pediatr 11(2):249–259
Deshmukh M, Patole S (2021) Prophylactic probiotic supplementation for preterm neonates-a systematic review and meta-analysis of nonrandomized studies. Adv Nutr 12(4):1411–1423
Yeung CY et al (2021) Immune modulation effects of lactobacillus casei variety rhamnosus on enterocytes and intestinal stem cells in a 5-fu-induced mucositis mouse model. Gastroenterol Res Pract 2021:3068393
Wu H et al (2020) Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 11(4):997–1014
Gong W et al (2016) Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis 7(9):e2387
Knudsen KBK et al (2017) Laser speckle contrast imaging to evaluate bowel lesions in neonates with NEC. Eur J Pediatr Surg Rep 5(1):e43–e46
Maki AC et al (2012) Intestinal microcirculatory flow alterations in necrotizing enterocolitis are improved by direct peritoneal resuscitation. Am Surg 78(7):803–807
Nowicki PT, Nankervis CA (1994) The role of the circulation in the pathogenesis of necrotizing enterocolitis. Clin Perinatol 21(2):219–234
Gonzalez LM et al (2019) Preservation of reserve intestinal epithelial stem cells following severe ischemic injury. Am J Physiol Gastrointest Liver Physiol 316(4):G482–G494
Nigmatullina L et al (2017) Id2 controls specification of Lgr5(+) intestinal stem cell progenitors during gut development. EMBO J 36(7):869–885
Drucker NA et al (2018) Stem cell therapy in necrotizing enterocolitis: current state and future directions. Semin Pediatr Surg 27(1):57–64
Drucker NA et al (2019) Inhibiting hydrogen sulfide production in umbilical stem cells reduces their protective effects during experimental necrotizing enterocolitis. J Pediatr Surg 54(1168):1173
Doster DL et al (2016) Mesenchymal stromal cell therapy for the treatment of intestinal ischemia: defining the optimal cell isolate for maximum therapeutic benefit. Cytotherapy 18(12):1457–1470
Markel TA et al (2015) Human mesenchymal stromal cells decrease mortality after intestinal ischemia and reperfusion injury. J Surg Res 199(1):56–66
Markel TA et al (2020) Human mesenchymal stem cell hydrogen sulfide production critically impacts the release of other paracrine mediators after injury. J Surg Res 254:75–82
Markel TA et al (2008) Stem cells as a potential future treatment of pediatric intestinal disorders. J Pediatr Surg 43(11):1953–1963
Drucker NA et al (2018) Hydrogen sulfide provides intestinal protection during a murine model of experimental necrotizing enterocolitis. J Pediatr Surg 53(9):1692–1698
Weil BR et al (2009) Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery 146(2):190–197
Funding
This study was funded by K08DK113226 from the National Institutes of Health, the George H. Clowes Memorial Research Career Development Award, the Riley Children’s Foundation, the Gerber Foundation, and the Department of Surgery at the Indiana University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TAM receives consulting fees from Noveome Biotherapeutics. There is no direct conflict with the information presented in this manuscript.
Ethical approval
All animal work in this study followed an IACUC-approved protocol at the Indiana University School of Medicine.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Primers (5′ → 3′).
Primer | Primer sequences |
|---|---|
GPR49-1 | CGACAACCACTACCTGAGCA |
GPR49-2 | CGGGACCAGATGCGATA |
GPR49-3 | AGCTAGGCTCTGCTCTGTCA |
Bmi1 | TAGACTTTTCTCGAGGTTTTCATGGTGTTACCTAAGACAAAAGACATCTCACCCTCTATGATGGACTTACTTCTGAGAGTGCGTTTGAGGCACTTATGGCTTACTAAGCAGTGTGTCACCATACTTGAAAACACTTCCATTTA TTGTATCTGGGATGAGGCTTTTTACCCTTACTCAATTTGA AAATTGC TTAAGCTTAAATGATATTTCAGTCAAAATTTGTCTTTTAATAAAACAACAGAAAGATG |
GAPDH | AGCTCCCCCCCACCATCCGGGTTCCTATAAATACGGACTGCAGCCCTCCCTGGTGCTCTCTGCTCCTCCCTGTTCCAGAGACGGCCGCATCTTCTTGTGCAGTGCCAGCCTCGTCCCGTAGACAAAATGGTGAAGGTCGGT GTGAACGGATTTGGCCGTATTGGGCGCCTGGTCACCAGGGCTGCCA TTTGCAGTGGCAAAGTGGAGATTGTTGCCATCAACGACCCCTTCAT TGACCTCAACTACATGGTCTACATGTTCCAGTATGACTCCACTCACGG CAAATTCAACGGCACAGTCA |
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosfield, B.D., Shelley, W.C., Mesfin, F.M. et al. Age disparities in intestinal stem cell quantities: a possible explanation for preterm infant susceptibility to necrotizing enterocolitis. Pediatr Surg Int 38, 1971–1979 (2022). https://doi.org/10.1007/s00383-022-05257-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05257-1