Abstract
Background
The aim of this study was to evaluate the swallowing problems by fiberoptic endoscopic evaluation of swallowing (FEES) study in both short- and long-gap patients after esophageal atresia (EA) repair.
Methods
Hospital records of patients who had undergone surgery for EA were reviewed retrospectively. Patients were divided into two groups as short-gap (SG) group (n:16) and long-gap (LG) group (n:10) to compare the swallowing problems. FEES study was performed, and the results were discussed in detail.
Results
There were twenty-six (16 M/10 F) patients with a mean age at evaluation was 7.52 ∓ 3.68 years. Mean follow-up period was 75.35 ∓ 44.48 months. In FEES study, pharyngeal phase abnormalities were detected in 10 patients (38.4%). Pharyngeal phase abnormalities were detected significantly higher in LG group (p:0.015). Laryngeal penetration/aspiration was seen in four patients on FEES study (15.3%). All of them was in LG group (40%). Laryngeal penetration/aspiration was seen significantly higher in LG group (p:0.014).
Conclusion
This is the first study to conduct FEES study in children after esophageal atresia repair to evaluate their swallowing conditions. Even though our sample is small, swallowing problems are more common than expected in the cases of LG when compared to SG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term gastrointestinal system problems such as gastroesophageal reflux (GER), dysphagia, and motility disorders could be observed frequently after esophageal atresia (EA) repair [1, 2]. Dysphagia, described as swallowing disorders induced by anatomic factors, sensory–motor or motor dysfunctions, is a common problem in patients after esophageal atresia repair [1, 3]. ESPGHAN and NASPGHAN guidelines reported that dysphagia incidence varies between 21 and 84% [4]. However, literature demonstrated that patients usually do not express dysphagia symptoms unless they are specifically asked [5]. Nevertheless, atresia patients should be evaluated for dysphagia. Videofluoroscopy swallowing study (VFSS) and fiberoptic endoscopic evaluation of swallowing (FEES) study could be employed to identify dysphagia [6]. Dysphagia screening and management are usually neglected by physicians [7]. Arslan et al. reported that only 19.4% of pediatric surgeons employed a standard dysphagia screening program after esophageal atresia repair [7]. Despite this, the majority of pediatric surgeons considered that early detection and treatment provide increased quality of life among atresia patients [7].
Oropharyngeal and esophageal phase abnormalities lead to swallowing disorders [8]. In the literature, a higher number of studies discussed esophageal phase abnormalities such as stricture, esophageal dysmotility when compared to those on oropharyngeal problems [3, 9]. However oropharyngeal phase abnormalities are a prerequisite for dysphagia in atresia patients with swallowing problems. Recently, VFSS, known as the gold standard in dysphagia, has been used more frequently when compared to FEES by surgeons in dysphagia diagnosis [10, 11]. Oral, pharyngeal, and esophageal phases could be evaluated with VFSS, but radiation exposure is a big drawback in this technique [6]. Langmore SE et al. published the first comparative study for FEES and VFSS in adult patients [12]. They reported that FEES had high specificity and sensitivity, especially for laryngeal penetration and aspiration [12]. To conclude, they proved FEES is a reliable technique to detect some of the major symptoms of dysphagia in the pharyngeal stage [12]. Kelly et al. conducted a retrospective study with 15 dysphagic patients’ FEES and VFSS records. The records were evaluated by speech-language pathologists. They rated higher PAS scores with FEES records than VFSS [13]. However, FEES can be challenging in the pediatric population because of discordance. In 1995, Willging P reported the first pediatric utility of FEES [14]. Recently, FEES is suggested by authors to evaluate swallowing conditions in esophageal atresia patients [6]. However, there is no data result of fiberoptic endoscopic evaluation in atresia patients in the literature.
Certain contributing factors such as anastomotic stricture, esophageal dysmotility, and structural airway malformation to dysphagia in esophageal atresia patients have been identified in the literature [4, 15, 16]. Baxter et al. reported that long-gap esophageal atresia was among the contributing factors to dysphagia [16]. They emphasized that esophageal atresia patients with a long gap had significantly lower functional oral intake scale scores [16]. Also, they reported that this could be associated with late-onset of oral feeding in long-gap atresia [16]. Similarly, our hypothesis premised that long-gap atresia patients have further oropharyngeal abnormalities in swallowing.
The aim of the current study was to evaluate oropharyngeal phase problems in esophageal atresia patients with FEES, and to compare the findings for the patients with long- and short-gap esophageal atresia.
Methods
The present study was conducted in pediatric surgery, otolaryngology, physical medicine, and rehabilitation departments in compliance with international ethical standards and the World Medical Association Declaration of Helsinki. The study was approved by the local Ethical Committee and an informed consent form was signed by all participants (Approval No: 21-11.1 T/21).
Hospital records of the patients who underwent esophageal atresia (EA) repair in our institution between the years January 2000 and January 2020 were reviewed retrospectively. The demographics, esophageal atresia type (long gap/short gap), and operation techniques (primary anastomosis or esophageal replacement) were detailed based on the hospital charts. If ‘the gap’ between the proximal and the distal pouch of the esophagus was too big to repair with primary anastomosis, it was classified as ‘long-gap atresia’. Patients who could be reached by phone call were subjected to a phone survey. The phoned survey was performed with parents. They were asked the following questions: “does he/she struggle when eating? Does he/she cough when he/she eats? Does it hurt when she/he eats? Does he/she have lung infections? If he/she has, how many times in a year? Also, physical evaluation was performed when patients came to the hospital for FEES.
FOIS
The feeding status of the patients was determined with the Functional oral intake scale (FOIS). Functional oral intake was classified based on the seven items in FOIS. The first three levels are associated with varying degrees of non-oral feeding, and the levels between 4 and 7 are associated with the patient’s oral food or liquid intake status [17].
FEES procedure
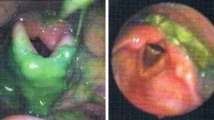
A FEES was performed using a Kaypentax Ltd, Montvale, NJ, USA, and Olympus, 1.8 mm diameter fiberoptic endoscope. A form was created to systematically record the FEES findings.
The FEES was administered in an upright seated position. This was done without administering topical anesthesia to the nasal cavity and video recordings were obtained for each patient. A flexible fiberoptic endoscope was used during the procedure.
The test protocol included two administrations of 3 ml, 5 ml, and 10 ml of water colored with food dye (green) via an injector. Similarly, swallowing tests were carried out using two administrations of one dessertspoonful of yogurt (5 ml) colored with food dye and fish crackers. Cleaning of the colored food was provided by making the patients drink water when the residue was detected during the test. We used fish crackers for solid food in order to obtain standard data. Velopharyngeal insufficiency, movement of the vocal folds, any delay in the onset of swallowing, premature spillage, retention-pooling, penetration, aspiration, and reflex coughing were all evaluated.
PAS
A Penetration–Aspiration Scale was used to describe aspiration events [18]. Eight points were used to define the aspiration status. The first five levels (1–5) describe penetration, which means materials that pass into the larynx but do not pass below the vocal folds. Levels 6–8 show aspiration, which means materials that pass below the vocal folds [18].
An ear, nose, and throat (ENT) specialist and a physical therapy and rehabilitation specialist were present during each of the procedures which were all video-recorded. All the test results were assessed by the same physiatrician, who was experienced and specialized in the topic, and the same ENT specialist.
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences version 21.0 software for Windows (IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp., USA). Patients’ characteristics and clinical parameters were assessed for normality with “Kolmogorov–Smirnov” and “Shapiro–Wilk” Tests. Univariate analyses of the variables in the study were performed using “Fisher Exact Test”, “Fisher-Freeman-Halton Exact Test”, and “Independent t-test”.
Twenty-six patients who were reached and allowed the FEES procedure were included in the study. The presence of residue, penetration, and aspiration during the FEES were recorded and analyzed.
Results
There were 117 patients who underwent esophageal atresia repair in the study period. We could not find the current phone number for 67 patients. There were fifty patients who could be reached by phone call (Fig. 1). However, 23 parents did not want to participate in the survey due to various reasons such as the distance between their residences and the hospital, and excessive fear of hospitals among the children. The participants are summarized in Fig. 1. Non-volunteering patients were excluded from the study. Twenty-seven (17 M/10 F) parents participated in the phone call survey and allowed the FEES study. Parents answered the questions. A ten-year-old boy, who underwent primary anastomosis, could not tolerate the FEES procedure. This patient was excluded from the study due to discordance. Eventually, 26 patients were included in the study.
The mean age of the patients was 7.52 ∓ 3.68 (7 months–15 years) at the time of the analysis. Sixteen male (61.5%) and ten female (38.5%) participants were included in the study. Most patients were in the short-gap group who underwent primary anastomosis (16 patients, 61.5%), and the remaining 10 patients were in the long-gap group who were treated with esophageal replacement (38.5%) (Gastric pull-up: 8, Colonic interposition: 2). The mean age was 8.22 ∓ 3.39 in the primary anastomosis group, and 6.40 ∓ 4.03 in the esophageal replacement group (p: 0.227). There were no statistically significant differences between the groups based on age and gender (Table 1). The mean follow-up period since the operation for these patients was 75.35 ∓ 44.48 months (7–137 months). The mean follow-up period for each group was mentioned in Table 1.
In the phone survey, according to what their parents answered, none of the patients complained of any dysphagia symptoms. They did not express any problem with oral feeding. Only two patients complained of recurrent lung infections. These two patients had pulmonary infections two or three times in a year. One of them was in the SG group (6.25%) and the other one in the LG group (10%).
Functional Oral Intake Scale (FOIS) score was 7 in 20, 6 in 4, and 5 in 2 patients, demonstrating that 20 patients had no swallowing problems. The remaining six patients experienced swallowing problems, four were fed totally orally with certain limitations of food, and two required some preparation. The rate of swallowing problems was 25% (4/16) in the SG group and 20% (2/10) in the LG group. The difference was not statistically significant between the two groups (p: 0.803).
There were no complications associated with FEES during or after the study. During FEES, oropharyngeal problems—residue in the retrocricoid region or piriform sinus or pharyngeal wall—were observed in ten patients. Seven of these ten patients were in the LG group (70%, 7/10) and three patients were in the SG group (18.8%, 3/16) (Table 2). Oropharyngeal problems were statistically higher in the LG group (p: 0.015).
Penetration and aspiration were determined with the Penetration–aspiration Scale in FEES. Twenty-two patients experienced no penetration or aspiration (84.6%). One patient had a penetration score of 5. The aspiration scores of 3 patients were 6 (material at a subglottic level without residue). All these patients were in the LG group (40%, 4/10). Sixteen patients in the SG group (100%) and six in the LG group (60%) experienced no penetration or aspiration (Table 2). Aspiration–penetration rate was statistically more common in the LG group (p: 0.014). All patients could tolerate the procedure and experienced no complications during the procedures.
Discussion
Feeding and swallowing disorders (SD) are common in infants and children after esophageal atresia repair. Despite the high prevalence of SD in EA patients, there are limited studies in the literature on feeding difficulties experienced by these children [8, 19]. While several studies focused on esophageal abnormalities as the source of feeding difficulties, oropharyngeal dysfunction and aerodigestive abnormalities should also be considered [4, 20, 21].
Mostly, patients do not recognize dysphagia symptoms unless these are specifically mentioned [5]. Our patient never complained about these symptoms during the phone surveys. Patients with esophageal atresia do not recognize dysphagia due to adaptation to their situation. But when they are asked specifically, it was observed that they experienced certain swallowing problems [4]. Thus, the EA patients should be routinely screened for early diagnosis of dysphagia [7].
In the pediatric literature, the Functional Oral Intake Scale was applied to esophageal atresia patients to determine the prevalence of dysphagia [22]. 111 patients were evaluated with FOIS and reported that dysphagia was prevalent across esophageal atresia patients, especially the young patients [22]. In this study, they modified this scoring system for infants [22]. It was reported that this modified system has adequate reliability and validity in infants [23]. In 2020, Yi et al. modified this scale into a 5-point scale and it showed adequate validity for children [24]. In our series, according to the Functional Oral Intake Scale, 76.9% of patients had total oral diet with no restriction (score: 7). Six patients (23%) had an oral diet with some specific limitation or special food restrictions (score: 5 or 6). Four of them were included in the SG group (25%) and 2 patients in the LG group (20%).
Oropharyngeal dysphagia with aspiration could be diagnosed objectively with several diagnostic tests. While there is no true gold standard to determine aspiration, all testing modalities are considered complementary [25]. We preferred FEES in the current study. Fiberoptic endoscopic evaluation of swallowing is an easy, well-tolerated, repeatable, and low-cost diagnostic method [12]. Unlike VFSS, there is no radiation exposure. Patients could be safely examined several times during both the preoperative and postoperative periods to determine the operation results. The procedure could be repeated several times to demonstrate progress. Another advantage of FEES is the real-time visualization of pharyngeal secretions [26,27,28].
There were some literatures which compared the findings of FEES and VFSS in dysphagia patients [28, 29]. FEES showed higher specificity for laryngeal penetration or aspiration compared to VFSS [28]. Furthermore, it was reported that abnormal findings were more common in FEES when compared to VFSS [29].
The potential complications of FEES are epistaxis, vasovagal syncope, and laryngospasm [26,27,28]. Thottom et al. reported 85 pediatric patients underwent endoscopic evaluation with no adverse effect [30]. Haller et al. displayed that there is no severe adverse event with FEES. However, especially younger children could not cooperate with FEES because of excessive crying [31]. They reported that excessive crying is the main problem for FEES [31]. Only one patient could not complete the procedure due to discordance in our series, while all other patients underwent the test easily, and no complication was observed in any patient.
Yalçın et al. indicated pharyngeal phase abnormalities in 28.2% of esophageal atresia patients with VFSS [10]. Coppens et al. evaluated 12 EA patients with VFSS and reported that 75% had pharyngeal phase abnormalities such as residue in the vallecula, piriform sinus, and pharyngeal wall [22]. 19 esophageal atresia patients were evaluated with VFSS and reported that aspiration was identified in the pharyngeal phase in 37% of the patients [32]. Thus, the authors suggested that motor dysfunction in the oropharyngeal phase was one of the main causes of aspiration [32]. Oropharyngeal abnormalities were common in our series, consistent with the literature. Ten patients (%38.4) presented pharyngeal phase abnormalities, and 7 were in the long-gap atresia group. Oropharyngeal abnormalities were more prevalent in the LG group.
In another study, 32 EA patients who underwent primary anastomosis were evaluated with VFSS [10]. No aspiration or penetration was indicated in 81.3% of patients. Five patients suffered aspiration based on PAS [10]. Four patients with aspiration on VFSS did not complain of recurrent lung infection. In our series, no patients indicated laryngeal aspiration or penetration in the SG group; however, 40% (n: 4) of patients in the LG group indicated aspiration or penetration. Similar to the pharyngeal residue, aspiration or penetration was more common in the LG group. One of these four patients experienced recurrent lung infections (10%). Three had no cough or pulmonary infection complaints. Thus, it could be suggested that aspiration or penetration could be present in esophageal atresia patients without clinical complaints.
Oropharyngeal dysphagia symptoms were more common than the complaints in esophageal atresia patients, especially among those with long-gap atresia. Consistent with our findings, it was reported in the literature that long-gap atresia was a dysphagia risk factor [16]. A retrospective study was conducted to determine dysphagia risk factors in long-term follow-up. They evaluated dysphagia outcomes based on the functional oral intake scale and reported that dysphagia was more common in long-gap esophageal atresia patients [16]. Oropharyngeal dysfunction was more common in long-gap esophageal atresia patients in the current study based on FEES.
To our knowledge, this is the first study in English literature on the employment of FEES in esophageal atresia cases. Although our patient series was small, we concluded that swallowing problems were prevalent in children with long-gap atresia. FEES was suggested by several studies to evaluate dysphagia in atresia patients; however, no clinical data is available. In our clinical study, the patients were evaluated with FEES to determine oropharyngeal abnormalities. It was determined that FEES was a reliable and repeatable method since it entails no radiation exposure.
The first limitation of the study is the limited patient number. We could not reach 67 patients due to missing phone numbers. Considering the time elapsed after the operation, the phone numbers of the patients may have changed, or they may have been registered incorrectly in the system. In addition, a few years ago, archival documents were transferred to the electronic system in our hospital.
The transfer to the new electronic patient file system continues. Also, esophageal atresia patients must be admitted to the hospital repeatedly and require some interventions for many years. So, they feel uncomfortable in the hospital and do not want to be admitted to the hospital if there is no emergency. Therefore, many patients do not want to participate in this study. Also, we had many patients from cities around the country, who could not come to the hospital easily. However, in our opinion, although the patients’ series was small, this study mentions very essential issues for esophageal atresia patients. Another limitation is the fact that the long-gap and short-gap patients have undergone different corrective procedures. Also, they have started oral feeding at different months of age. Therefore, it would be considered these factors affect oropharyngeal phase abnormalities. Also, the long-term follow-up period is not similar between the two groups since the short-gap group has a longer follow-up period. We considered that swallowing functions may get better in time; therefore, the long-gap group would be re-controlled with FEES in the future. Another limitation is the FOIS scoring system. We did not use the pediatric version of the scale. We did not use the new version as we evaluated all our patients before publishing the new validated version. However, despite all, this study is important to draw surgeons’ attention to swallowing problems in esophageal atresia.
To conclude, esophageal atresia patients would not experience normal swallowing motility, and they commonly do not complain of dysphagia. Thus, they should be evaluated for dysphagia even when they do not have complaints. These patients require a screening program and prolonged multidisciplinary follow-up to determine swallowing disorders. FEES is a reliable and easy method for the diagnosis of oropharyngeal dysphagia. In our opinion, the current study is important in emphasizing dysphagia and the clinical application of FEES.
Abbreviations
- EA:
-
Esophageal atresia
- FEES:
-
Fiberoptic endoscopic evaluation of swallowing
- FOIS:
-
Functional oral intake scale
- GER:
-
Gastroesophageal reflux
- PAS:
-
Penetration–aspiration scale
- VFSS:
-
Videofluoroscopy swallowing study
References
Rintala RJ, Pakarinen MP (2013) Long-term outcome of esophageal anastomosis. Eur J Pediatr Surg 23:2019–2025. https://doi.org/10.1055/s-0033-1347912
Tuğcu GD, Soyer T, Polat SE et al (2021) Evaluation of pulmonary complications and affecting factors in children for repaired esophageal atresia and tracheoesophageal fistula. Respir Med 181:106376. https://doi.org/10.1016/j.rmed.2021.106376
Rayyan M, Allegaert K, Omari T et al (2015) Dysphagia in children with esophageal atresia: current diagnostic options. Eur J Pediatr Surg 25:326–332. https://doi.org/10.1055/s-0035-1559818
Krishnan U, Mousa H, Dall’oglio L et al (2016) ESPGHAN-NASPGHAN guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with esophageal atresia-tracheoesophageal fistula. J Pediatr Gastroenterol Nutr 63:550–570. https://doi.org/10.1097/MPG.0000000000001401
Lemoine C, Aspirot A, Le Henaff G et al (2013) Characterization of esophageal motility following esophageal atresia repair using high-resolution esophageal manometry. J Pediatr Gastroenterol Nutr 56:609–614. https://doi.org/10.1097/MPG.0b013e3182868773
Mahoney L, Rosen R (2016) Feeding difficulties in children with esophageal atresia. Pediatr Respir Rev 19:21–27. https://doi.org/10.1016/j.prrv.2015.06.002
SerelArslan S, Demir N, Karaduman AA et al (2021) Dysphagia in children with EA-TEF from the perspective of pediatric surgeons in clinical settings. Dysphagia 36:644–649. https://doi.org/10.1007/s00455-020-10178-z
Conforti A, Valfre L, Falbo M et al (2015) Feeding and swallowing disorders in esophageal atresia patients: a review of a critical issue. Eur J Pediatr Surg 25:318–325. https://doi.org/10.1055/s-0035-1559819
Cartabuke RH, Lopez R, Thota PN (2016) Long-term esophageal and respiratory outcomes in children with esophageal atresia and tracheoesophageal fistula. Gastroenterol Rep 4:310–314. https://doi.org/10.1093/gastro/gov055
Yalçın S, Demir N, Serel S et al (2015) The evaluation of deglutition with videofluoroscopy after repair of esophageal atresia and/or tracheoesophageal fistula. J Pediatr Surg 50:1823–1827. https://doi.org/10.1016/j.jpedsurg.2015.07.002
Demir N, Arslan SS, Yalcin S et al (2017) Alterations in hyolaryngeal elevation after esophageal anastomosis: a possible mechanism for airway aspiration. J Pediatr Surg 52:1580–1582. https://doi.org/10.1016/j.jpedsurg.2017.04.001
Langmore SE, Shatz K, Olson N (1991) Endoscopic and videofluoroscopic evaluation of swallowing and aspiration. Ann Otol Rhinol Layrngol 100:678–681. https://doi.org/10.1177/000348949110000815
Kelly AM, Drinnan MJ, Leslie P (2007) Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope 117:1723–1727. https://doi.org/10.1097/mlg.0b013e318123ee6a
Willging JP (1995) Endoscopic evaluation of swallowing in children. Int J Pediatr Otorhinolaryngol 32:S107–S108. https://doi.org/10.1016/0165-5876(94)01174-v
Castilloux J, Noble AJ, Faure C (2010) Risk factors for short-and long-term morbidity in children with esophageal atresia. J Pediatr 156:755–760. https://doi.org/10.1016/j.jpeds.2009.11.038
Baxter KJ, Baxter LM, Landry AM et al (2018) Structural airway abnormalities contribute to dysphagia in children with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg 53:1655–1659. https://doi.org/10.1016/j.jpedsurg.2017.12.025
Crary MA, Carnaby Mann GD, Groher ME (2005) Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 86:1516–1520. https://doi.org/10.1016/j.apmr.2004.11.049
Rosenbeck JC, Robbins JA, Roecker EB et al (1996) A penetration-aspiration scale. Dysphagia 11:93–98. https://doi.org/10.1007/BF00417897
Marinschek S, Pahsini K, Aguiriano-Moser V et al (2020) Efficacy of a standardized tube weaning program in pediatric patients with feeding difficulties after successful repair of their esophageal atresia/tracheoesophageal fistula. Eur J Pediatr 179:1729–1737. https://doi.org/10.1007/s00431-020-03673-w
Spitz L (2006) Esophageal atresia. Lessons I have learned in a 40-year experience. J Pediatr Surg 41:1635–1640. https://doi.org/10.1016/j.jpedsurg.2006.07.004
Rommel N, Rayyan M, Scheerens C et al (2017) The potential benefits of applying recent advances in esophageal motility testing in patients with esophageal atresia. Front Pediatr 5:137. https://doi.org/10.3389/fped.2017.00137
Coppens CH, van den Engel-Hoek L, Scharbatke H et al (2016) Dysphagia in children with repaired oesophageal atresia. Eur J Pediatr 175:1209–1217. https://doi.org/10.1007/s00431-016-2760-4
Yi YG, Shin HI (2019) Psychometrics of the functional oral intake scale for infants. Front Pediatr 7:156. https://doi.org/10.3389/fped.2019.00156
Yi YG, Shin HI (2019) Psychometrics of the functional oral intake scale for children with dysphagia. J Pediatr Gastroenterol Nutr 71:686–691. https://doi.org/10.1097/mpg.0000000000002861
Mahoney L, Rosen R (2017) Feeding problems and underlying mechanism in the esophageal atresia -Tracheoesophageal fistula patient. Front Pediatr 5:127
Langmore SE (2017) History of fiberoptic endoscopic evaluation of swallowing for evaluation and management of pharyngeal dysphagia: changes over the years. Dysphagia 32:27–38. https://doi.org/10.1007/s00455-016-9775-x
Beer S, Hartlieb T, Müller A et al (2014) Aspiration in children and adolescents with neurogenic dysphagia: comparison of clinical judgment and fiberoptic endoscopic evaluation of swallowing. Neuropediatrics 45:402–405. https://doi.org/10.1055/s-0034-1387814
Da Silva AP, LubiancaNeto JF, Santoro PP (2010) Comparison between videofluoroscopy and endoscopic evaluation of swallowing for the diagnosis of dysphagia in children. Otolaryngol Head Neck Surg. 143:204–9. https://doi.org/10.1016/j.otohns.2010.03.027
Pavithran J, Puthiyottil IV, Kumar M, Nikitha AV, Vidyadhaaran S, Bhaskaran R et al (2020) Exploring the utility of fiberoptic endoscopic evaluation of swallowing in young children–a comprasion with videofluoroscopy. Int J Pediatr Otorhinolaryngol 138:110339. https://doi.org/10.1016/j.ijporl.2020.110339
Thottam PJ, Silva RC, McLevy JD et al (2015) Use of fiberoptic endoscopic evaluation of swallowing (FEES) in the management of psychogenic dysphagia in children. Int J Pediatr Otorhinolaryngol 79:108–110. https://doi.org/10.1016/j.ijporl.2014.11.007
Haller L, Osterbauer B, Maldonado K, Bhardwaj V, Bansal M, Peck K et al (2020) Factors impacting participation in flexible endoscopic evaluation of swallowing in children. Int J Pediatr Otorhinolaryngol 138:110323. https://doi.org/10.1016/j.ijporl.2020.110323
Hörmann M, Pokieser P, Scharitzer M et al (2002) Videofluoroscopy of deglutition in children after repair of esophageal atresia. Acta Radiol 43:507–510. https://doi.org/10.1034/j.1600-0455.2002.430511.x
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to disclose
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Celtik, U., Eyigor, S., Divarci, E. et al. Fiberoptic endoscopic evaluation of swallowing (FEES) study: the first report in children to evaluate the oropharyngeal dysphagia after esophageal atresia repair. Pediatr Surg Int 38, 1227–1233 (2022). https://doi.org/10.1007/s00383-022-05169-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-022-05169-0