Abstract
Purpose
The use of intercostal nerve cryoablation (INC) is becoming increasingly common in patients undergoing pectus excavatum (PE) repair. This study sought to evaluate the use of INC compared to traditional use of thoracic epidural (TE).
Methods
A retrospective review of 79 patients undergoing PE repair with either INC or TE from May 2009 to December 2019 was conducted. The operations were performed by four surgeons who worked together at four different hospitals and have the same standardized practice. The primary outcome measure was hospital length of stay (LOS). Secondary variables included surgical time, total operating room time, operating room time cost, total hospital cost, inpatient opioid use, long-term opioid use after discharge, and postoperative complications.
Results
LOS decreased to 2.5 days in the INC group compared to 5 days in the TE group (p < 0.0001). Surgical time was increased in the INC group, but there was no difference in total OR time. The INC group experienced significantly lower hospital costs. Total hospital opioid administration was significantly lower in INC group, and there was a significant decrease in long-term opioid use in the INC group.
Conclusions
INC is a newer modality that decreases LOS, controls pain, and results in overall cost savings. We recommend that INC be included in the current practice for postoperative pain control in PE patients undergoing Nuss procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pectus excavatum (PE) is a congenital birth defect that is often noticeable shortly after birth, typically worsening during adolescence. PE occurs in 1 every 400–1000 births and is four times more prevalent in males than females [1]. The most often performed surgical technique to correct the deformity is the Nuss procedure. This procedure is associated with significant postoperative pain and is now considered to be the preferred surgical technique due to decreased procedure degree of difficulty, and improved cosmesis with smaller, less central scars [2].
Pain management has been an ongoing issue with PE repair, and surgical pain has traditionally been managed with the use of thoracic epidural (TE). Length of stays can approach a week [3]. Although TE is an effective analgesic modality, rare side effects include nausea, vomiting, pruritis, dizziness, hypotension, and neuropathy [4]. Alternatives to use of TE consist of intravenous patient-controlled analgesia, bilateral ultrasound-guided nerve blockade, paravertebral blocks (with or without indwelling catheters), and bupivicaine liposome injection suspension [5,6,7,8]. In patients undergoing PE repair, regional nerve block techniques have been notable for achieving variable degrees of improvement in length of stay (LOS) [9]. Regardless of the various regional techniques utilized for pain management post-PE repair, new multi-modal analgesic regimens are being developed to facilitate enhanced recovery after surgery (ERAS) [10, 11].
Intercostal nerve cryoablation (INC) is now demonstrating promising pain management results for pediatric patients undergoing PE repair. INC is being used without the use of TE resulting in shorter LOS [12]. This study sought to compare patients undergoing Nuss repair receiving INC versus TE and to assess differences in LOS. Additionally, this study looked at inpatient opioid use, surgical time, total operating room time, operating room time cost, total hospital cost, long-term opioid use after discharge, and postoperative complications.
Materials and methods
Retrospective review and data collection
This was a retrospective study of patients who underwent Nuss repair of PE with INC or TE for postoperative pain control. Forty of the most recent patients who underwent INC were compared with matched controls from the past 10 years. Patients who received INC underwent surgery from July 9, 2017 to December 20, 2019 and those that received TE analgesia underwent PE surgery from May 1, 2009 to November 23, 2016. Only patients who received INC or TE were included in this study; study subjects were identified via search using the International Statistical Classification of Diseases and Related Health Problems ICD-9 and ICD-10 codes for diagnosis of PE. Patients who had received TE for pain control were matched with patients who had received INC. Matching was based upon age, sex, and number of bars.
Surgery was performed within an integrated (horizontal and vertical) health care system by four different surgeons at four Southern California Kaiser Permanente hospitals. Each surgeon had extensive experience performing the Nuss procedure. Surgical technique was standardized amongst all four surgeons, though the number of bar implants was left to the discretion of the operating surgeon. All surgeons switched from using TE to INC simultaneously, and there were no individual variations. Detailed demographics and perioperative characteristics were collected pertaining to hospital LOS, total surgical time, total operating room time, operating room time cost, total hospital cost, inpatient opioid utilization, long-term opioid use after discharge, and postoperative complications.
Patient demographic variables collected included age, gender, ethnicity, and number of surgical bars used. Preoperative CT scans were performed only in some patients; therefore, the Haller index was not routinely determined. Patients were deemed candidates based on the severity of their defect and/or symptoms that included shortness of breath, fatigue, or chest pain. Operative data, inpatient data, and outpatient follow-up data were collected. Additionally, subjective patient complaints were noted at the 2-week, 3-month, and 1-year follow-up appointments. Any procedure-related complications were reviewed and noted.
The primary outcome measure was LOS. Oral and intravenous opioid quantities were expressed in oral morphine milligram equivalents (Mmeq) [13]. Secondary outcome measures included surgical time, total time in the operating room, total operating room cost, total hospital costs, total inpatient opioid use, long-term opioid use after discharge, and postoperative complications.
Surgical repair pectus excavatum
Patients selected for the Nuss repair followed the technique outlined by Nuss et al. [14]. In brief, the deepest point of the sternal depression was determined and then the incisions were marked laterally, beginning at the anterior axillary line and continuing posteriorly for approximately 5 cm. Subcutaneous flaps were raised bilaterally. Limited flaps were raised under the pectoralis muscle. Using bilateral thoracoscopic visualization, the chest was penetrated with the dissector, which was then passed between the back of the sternum and the front of the pericardium. The tip of the dissector was then passed out through the corresponding intercostal space on the left side of the sternum. A strong suture was connected to the dissector, which was then pulled back out of the chest from left to right. An appropriately sized bar was then pre-bent and pulled into the chest from right to left under direct vision. The bar ends were adjusted with the bar bender as needed, and it was inverted into proper position. A second bar, placed thorough the same skin incision, was required for longer and more severe defects. The ends of the bars were connected to stabilizers to prevent postoperative bar migration. The bars were sutured to the muscle using several interrupted long-term absorbable sutures. The air was evacuated from the chest and the wounds were closed in layers with absorbable sutures.
Intercostal nerve cryoablation
When INC was utilized, it was performed under direct thoracoscopic visualization using the cryoICE™ (AtriCure, Minnesota, USA) device before beginning the dissection for the minimally invasive repair. Working under the developed subcutaneous skin flaps, the probe was applied externally, inserting it through the chest wall and intercostal muscles at the neurovascular bundle along the inferior aspect of the ribs. Once the tip of the probe was visualized in a subpleural location, a cooling temperature of negative 60 degrees centigrade was applied through the probe for 2 min, from the third through seventh intercostal spaces bilaterally. Care was taken to avoid ablating cephalad to the third and caudad to the ninth intercostal spaces. The probe adhered to the tissue while freezing, and it needed time to thaw (15 s being sufficient in most applications) and separate from the tissue prior to moving it to the next interspace. Local anesthetic block (0.25% bupivacaine with epinephrine) was infiltrated into all incisions at the end of the case.
Thoracic epidural pain management
Thoracic epidurals were placed into the T5–T6, T6–T7, or T7–T8 interspace based upon anatomic assessment by each attending anesthesiologist. The thoracic epidurals were all placed in the operating room by an anesthesiologist. The specifics of the procedure were left to the discretion of the attending anesthesiologist. Prior to incision, the epidural was activated with 5–10 mL of bupivacaine 0.25% based upon the patient’s weight. Epidural analgesia was maintained throughout the procedure with bupivacaine 0.125–0.25% mixture at a rate of 5–8 mL/h. Local anesthetic block (0.25% bupivacaine with epinephrine) was infiltrated into all incisions at the end of the case. In the post-anesthesia care unit (PACU), the epidural solution was changed to 0.125% bupivacaine with 20 mcg/mL hydromorphone at a rate of 7 mL/h. Patients in the INC group did not receive a TE, and patients in the TE group did not receive INC.
Postoperative pain care for INC patients
In the INC group, patients received a postoperative pain management regimen consisting of hydromorphone PCA (0.2 mg every 10 min demand dose without continuous dose) beginning on the day of surgery (i.e. postoperative day 0). The patients were subsequently transitioned to hydrocodone-acetaminophen 5/325 mg every 4 h as needed for pain as well as a non-steroidal anti-inflammatory drug (ibuprofen or ketorolac, depending on clinical impression), and gabapentin depending on surgeon preference. Intravenous breakthrough opiates were given if patients continued to have severe pain based upon nursing discretion. All patients were admitted to a pediatric floor postoperatively. Per institutional protocol, all epidurals were weaned at the discretion of the pediatric pain management team in consultation with the primary surgical team, usually on postoperative day (POD) 2 or 3.
Discharge readiness
Patients were determined to be ready for discharge after meeting the discharge criteria. This consisted of the following: no major pneumothorax seen on chest X-ray, able to tolerate food by mouth, pain adequately controlled on oral pain medications, able to urinate spontaneously, and able to pass physical therapy evaluation which included ambulating without assistance and ability to perform activities of daily living. Additionally, the patients and families were provided with a packet upon discharge that detailed activity restrictions, side effects of medications, what to expect at home postoperatively, and instructions to contact pediatric surgery office or return to the emergency department if certain symptoms arose while at home.
Follow-up
Patients were sent home with acetaminophen, oxycodone, or hydrocodone, and scheduled for follow-up appointments at 2 weeks, 3 months, and 1 year from the date of their discharge from the hospital. Physicians performed a focused physical exam and obtained a detailed history during these visits. Incisional sites, epidural catheter sites, and the alignment of the patient’s Nuss bars were assessed at these visits.
Institutional cost structure
The costs for operating room time and hospital days were based upon an estimate of our system’s cost per Kaiser Foundation Health Plan and Southern California Permanente Medical Group based upon fiscal year 2018. Services reflect allocation of direct costs only; fixed costs, such as depreciation, hospital administration, and insurance are not included in the cost accounting analysis. Total hospital cost was approximated as the sum of the total operating room time costs, INC equipment costs or TE costs, and the cost of each hospital day.
Primary outcome measure
The primary outcome measure for this study was postoperative length of stay (LOS). LOS was calculated as the number of postoperative days in the hospital. At our various institutions, patients with thoracic epidurals would be admitted to the pediatric intensive care unit (PICU) for several days for TE management. Our institutions do not have a protocol for dealing with TEs on the floor, and thus there was a bias for having patients in the PICU in our study. A PICU day was instead counted like a floor day for purposes of removing bias.
Secondary outcome measures
Secondary outcome measures included surgical operative time, total operating room time, operating room time cost, total hospital cost, total hospital opioid utilization, long-term opioid use after discharge, and postoperative complications. Surgical operative time was defined as time from incision until closure. Total operating room time was defined as time patient entered the operating room until closure. Total hospital cost was the sum of costs for operating room time, INC costs or TE costs, and each hospital day costs. Professional fees for both surgeons and anesthesiologists were not included, however, in our health system, both the surgeon and anesthesiologist are compensated with a yearly base salary regardless of clinical complexity or intensity. The actual cost of a hospital day in Kaiser system is difficult to compute because this information is proprietary, and patients are not charged per day due to capitated payment arrangements per member per year. An estimate of cost per hospital day is between $2,000 and $6,000 per day [15, 16]. For this study, a conservative estimate of $2,000 per hospital day was used.
Institutional costs for INC were $2,400 per case. Thoracic epidural kit costs $22.30 each. Operating room time costs approximated $56.75 per minute. Total hospital opioid utilization was the sum of opioid consumption (oral and IV) during and after surgery while the patient remained in the hospital. Long-term opioid use after discharge was determined by the amount of opioids prescribed to the patient upon hospital discharge. At follow-up appointments and through nurse phone calls, patients were asked about specific amount of pills used and the need for refills.
Statistical analysis
All data were analyzed using an intention-to-treat analysis. The primary outcome data were analyzed by comparing the mean LOS with a two-tailed t test. The other continuous variables were analyzed by a two-tailed t test. Lastly, categorical data were compared using the test χ2 and Fisher’s exact test.
Results
This study evaluated 79 patients who underwent PE repair. Patients undergoing the Nuss procedure with INC (N = 40) were compared to historical control patients undergoing PE repair with TE (N = 39) during the study period. There were no differences between the groups in sex, age, and number of bars (see Table 1). However, there were significantly more Hispanic patients in the TE group (p < 0.02) and significantly more Caucasian patients in the INC group (p < 0.001). Mean follow-up in the TE group was 34.3 months, while follow-up in the INC group had a mean of 14.1 months. Twelve patients in the TE group presented with preoperative symptoms of chest pain, while 12 patients in the INC group also presented with preoperative symptoms of chest pain. Preoperative chest pain was not quantified. In the INC group, 42.5% of patients have had their bars removed, while 100% of TE patients have had their bars removed. Not all bars in the INC group have been removed because patients in the INC group underwent Nuss repair more recently and many have not reached their 2- to 3-year follow-up appointments. In the TE group, 97.4% of patients had their urinary catheters in for longer than a day, while none of the patients in the INC group had foley catheters in for longer than a day (p < 0.0001) (see Table 1).
Outcome measures
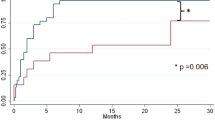
There was a shorter hospital LOS among patients in the INC group (average 2.5 days) compared to those in the TE group who averaged 5 days in the hospital (p < 0.0001) (see Table 2). The trend of LOS for patients over time as the surgeons completed more Nuss procedures was assessed. There was no correlation between the number of Nuss procedures surgeons performed in INC group and a decrease in LOS trend as technique improved (see Fig. 1). There was also no trend toward decreased LOS as the surgeons completed more Nuss procedures in the TE group (see Fig. 2).
There was no impact in LOS in either groups as the learning curve for the surgeons decreased. Additionally, the PICU LOS was shorter among patients who underwent INC compared to patients who received TE, though we did not include this in our secondary outcomes due to differences in protocol amongst institutions regarding sending these patients to the PICU.
Overall OR time was no different between the INC group, which averaged 151 min, and the TE group, which averaged 150 min (p = 0.88) (see Table 3). All of the TEs were placed in the OR (39 out of 39) before an incision was made. The time spent placing the epidurals before incision was similar to the time spent on cryoablation. Surgical operative times were longer in INC group (average 116 min) vs the TE group (average 80 min) because the INC was performed after the incisions were made (p < 0.0001). All patients had successful correction of PE.
There are increased initial costs associated with INC. Surgical times increased by 36 min and the cost of each minute of OR time at our institution is $56.75. This was offset by the time spent placing the epidurals in the operating room in the TE group. The cost of INC was $2,400 per case per institutional costs. On average, patients in the INC group had lower total hospital costs compared to those in the TE group (see Table 3). Total hospital cost average for TE group was $18,336, while total hospital cost average for INC group was $15,976 (p < 0.0005).
There was a significant difference between the amount of inpatient opioid used in the INC group (average of 111 Mmeq) vs the TE group (average of 269 Mmeq) (p < 0.0001) (see Table 3). Outpatient utilization of additional opioids was also statistically significantly less in the INC group (average of 205 Mmeq) compared to the TE group (average of 553 Mmeq) (p = 0.003) (see Table 3).
Patient-related complications
There were no major complications in the TE group. In the INC group, one patient required Nuss bar removal after 1 month due to intractable pain. This patient was an outlier because he was an older individual (28 years old) who wanted to have the Nuss procedure. There was no long-term neuropathy in either group. The INC group had one patient return to the emergency department (ED) and was readmitted for pain—attributed to forgetting to fill outpatient acetaminophen/paracetamol prescription. The TE group had one patient that returned to the ED and was readmitted due to uncontrolled postoperative pain after lifting 100 lb. He was found to have low-grade fevers and sent home with 4 weeks of antibiotics for an infection. The TE group had one patient that developed a hydropneumothorax requiring chest tube placement. Two patients in each group developed urinary retention postoperatively that improved after foley replacement and voiding trial. There were no instances of urinary tract infection or bleeding in either group. No study cohort required surgical reintervention for bar displacement. Two patients in the TE group had recurrence of PE after bar removal (see Table 4).
Discussion
The introduction of a new surgical nerve ablation technique employing cryoanalgesia to intercostal nerves was first described by Lloyd in 1976 [18]. Since its initial introduction, INC (cryoICE™, AtriCure, Minnesota, USA) has been studied in conjunction with and without the use of epidurals for the management of post-thoracotomy pain in multiple surgical populations [19, 20].
Postoperative pain management after the Nuss procedure has been the subject of much interest during the past few years, due to the difficulty managing pain, high degree of opioid utilization, and prolonged hospital LOS in these patients [21]. Initially, experience in our health care system with thoracic epidurals is that they provided satisfactory pain control for 2–3 days. Once patients were weaned off the epidural, they experienced continued pain and it took another 2–3 days to become manageable with oral therapy. Often, patients would go home with significant pain that took days to weeks to resolve. Consequently, multi-modal pain regimens and ERAS protocols were employed to improve LOS and pain control in patients. Some multi-modal pain regimens have also included paravertebral blocks [22].
However, the treatment paradigm changed with the description by Keller et al. of the new cryoanalgesia technique in 2016 [12]. Keller showed that the LOS in patients undergoing pectus repair with this novel technique was two days less than their TE group. They found that 62% of their INC patients left the hospital before day three compared to 4% of patients in the TE group [12]. Graves et al. reported similar findings. Their INC patients had a postoperative LOS that was significantly shorter than TE (2.0 ± 0.82 vs. 6.3 ± 1.3 days) [23]. A randomized control trial by Graves et al. showed that the median length of stay in INC patients decreased to 3 days from 5 days compared to their TE group [3]. Many pediatric surgery groups became interested in INC due to the compelling results of these papers. Because of this, our group instituted the use of INC over the past few years. Our group consisted of four surgeons who shared a standardized practice across four hospitals.
In this study, the hospital length of stay was shortened from 5 to 2.5 days in the INC group compared to the TE group. Sixty-five percent of patients in the INC group were discharged by POD 2 compared to zero percent in the TE group. Almost 90% of patients in the INC group were discharged by POD 3 compared to 5.1% of patients in the TE group. In both cohorts, the only variable keeping patients in the hospital was pain control. The patients in each group were matched, with an equal number presenting with preoperative chest pain. There were no differences in medical or surgical history between the two groups to bias the TE patients toward longer lengths of stay. Even with recent implementation of ERAS protocols with thoracic epidural patients in other studies, the LOS has been historically higher than INC groups due to issues with postoperative pain control [3, 12].
As each surgeon became more experienced with the INC technique, the LOS did not significantly trend down in either group. This showed that the learning curve did not have an impact on LOS. Additionally, there was no difference in LOS based on how recently the Nuss procedure had been performed in either the INC group or the TE group. One possible confounding variable of this study is the large time period between the TE patients and the INC patients. Extended periods of time can introduce bias. The most definitive way to answer this question would be a prospective randomized trial with long-term follow-up. However, over the 10-year time period for TE and INC cases, there were no other protocol changes in surgical management or decision making. All patients were sent home using the same discharge criteria.
In a prospective randomized trial by St. Peter et al., Nuss patients with epidurals had an average total hospital cost of $45,400 [6]. In an abstract by Weissler et al., 9032 patients who underwent PE repair were assessed for total hospital costs. The average hospital charge was $41,016, according to their study [17]. A study by Loftus et al. showed a total hospital cost average of $25,440 for TE patients, with the sum of OR, PACU, and floor costs being $18,637 [15]. The INC group experienced significantly decreased total hospital costs ($15,976) compared to the TE group ($18,335) (p < 0.0005) in our study and was also well below the costs of the TE groups in the above studies.
Since this study was performed at an integrated health care system (Kaiser Permanente Southern California), each hospital day may cost less than a hospital day at other hospitals. The cost of a regular hospital day within Kaiser Permanente is proprietary information and cannot be released. Thus, our study demonstrated a trend in cost savings, but the absolute dollar amount reduced cannot be extrapolated to other centers. In a study by Loftus et al., the cost per hospital day for patients undergoing PE surgery with TE was approximately $2,000 [15]. A recent study by Melnick et al. estimated that the cost of one hospital day in California averaged $5,735 [16]. In this study, a conservative estimate of $2,000 per day of hospitalization was used. If an estimate of $3,000–$6,000 per hospital day was used instead, the differences in cost savings between the INC group and TE group would be even more dramatic due to the significant decrease in LOS in the INC group.
Within the Kaiser Permanente integrated health care system, patients with TE often necessitated PICU admission and remained in bed (i.e. non-ambulatory) due to fall risk in accordance with nursing policies. When INC was introduced, it resulted in a significant reduction and often complete elimination of PICU admissions. This is even more relevant to cost analysis. The cost of one PICU day at Kaiser Permanente is higher than a regular hospital floor day. If the PICU cost data were included in the analysis, the total hospital costs for INC group compared to TE group would be further decreased. Decreasing the child’s LOS has a direct effect on parents as well, allowing parents to take less time off from work. This has economic implications for families as well. Thus, there are even more positive effects from using INC in PE patients than the data demonstrate in our study.
There are initial increased up-front costs associated with INC. Since overall operating room time was similar, the discrepancies in cost were due to the additional INC equipment fees. These increased costs are mitigated by a decreased hospital LOS (an average decrease of 2.5 days), resulting in significant overall cost reduction. There could be some savings in cost in the TE group if there was a protocol implemented that placed the epidurals outside the OR before the operation. In the current economic climate to curtail costs and conduct risk–benefit assessments into whether the cost of new technology is justifiable, our study provides new evidence that the use of INC leads to significantly reduced total hospital costs since it lowers overall LOS.
Patients often felt completely back to normal by the first postoperative visit at 2 weeks and wanted to return to sports and regular activities immediately. They had to be counseled to ease back into their normal routine for fear of bar displacement. Three of 26 patients in the INC group from the Keller study had bar displacement requiring reoperation [12]. It was hypothesized that since most of the pain sensation was removed after the procedure by INC, these patients were more inclined to ignore activity restrictions [12]. This did not occur in the patient population in our study. There was also a trend over time to place two bars for better cosmetic results in the INC group, which explains the significant difference in number of bars in each group.
The use of INC offers significant advantages regarding opioid consumption and utilization. The INC technique results in an anesthetic effect on the nerves [24] and decreases the need for opioids. Our study findings were consistent with Graves et al., who found cryoablation patients also required significantly less inpatient opioid analgesia [3]. Perhaps more importantly, since the INC group’s pain was so much better controlled after discharge, there was a significant decrease in long-term opioid use as well. This is particularly important in the current opioid crisis. There is a desire to limit the prescription of postoperative opioids due to the risk of dependency. The INC technique is opioid sparing and is an excellent alternative to narcotic use.
Overall, there were minimal postoperative complications in both cohorts. Our study cohorts did not sustain any issues with bar displacement requiring surgical intervention, and there was only one hospital readmission in each group due to pain. We believe this was due to detailed preoperative patient education coupled with close follow-up, prescriptive physical therapy, and active communication between the surgical team and the patient/family. There was also an initial concern about short-term and long-term neuropathy in these patients. We initially thought gabapentin was needed to mitigate this. A study by Zobel et al. looked at incidence of neuropathic pain after Nuss repair with INC. The study showed that no patients under the age of 21 (30 total) experienced neuropathic pain [25]. Our study was consistent with this literature. In the INC group, only two patients experienced neuropathic pain and were treated with gabapentin, but none of our study cohorts had any long-term numbness. We no longer prescribe empiric gabapentin.
There were several limitations in this study. The primary limitation was related to the lack of uniformity in the analgesic regimens utilized perioperatively between the two groups. The administration of acetaminophen, gabapentin, ketorolac, and ibuprofen was based upon both the surgeon and anesthesiologist’s preference, and there was no adherence to a formalized multi-modal analgesic protocol. Additionally, none of the patients who underwent TE for pain management received gabapentin. This reflects the lack of use of gabapentin when TE was considered standard of care prior to the implementation of enhanced recovery after surgery protocols coupled with use of INC at our integrated health system. Thus, we considered usage of gabapentin as a potential confounder and adjusted for it in our models. Despite the lack of uniform multi-modal regimens between and within the two groups, the INC group demonstrated decreased LOS, decreased overall hospital costs, decreased inpatient opioid utilization, and decreased outpatient opioid utilization.
We also elected to omit the epidural hydromorphone from the total hospital opioid use. The reason is that we were looking for oral/IV differences in opioids rather than epidural. Epidural opioids generally do not migrate systemically, and there is no standardized way to convert epidural hydromorphone to morphine milligram equivalents. However, taking epidural hydromorphone into consideration, one can see that the TE group did receive even more opioids than we present in this study. Finally, retrospective studies are often subject to variations in care that may be difficult to capture with data review.
Conclusion
Intercostal nerve cryoablation significantly decreased the LOS for patients undergoing the Nuss procedure for PE compared to our control group treated with TE. Our study shows significantly reduced overall hospital costs, reduced inpatient opioid use, reduced outpatient opioid use, and no difference in total operating room time. To date, we have not seen any long-term negative effects of INC, though we are continuing to monitor patients for long-term complications. The results of this study support the findings of the current literature, including the randomized controlled trial by Graves et al. [3]. The results of this study suggest that INC could be superior to TE use. Additional studies are needed to confirm these results with larger number of patients.
We recommend that all pediatric surgeons embrace this newer technology for postoperative pain management for patients undergoing Nuss procedures. We are optimistic that the use of INC will be beneficial in multiple other surgical populations, and we have started to integrate INC in other operations including Ravitch procedures and other thoracotomies.
References
Fokin AA, Steuerwald NM, Ahrens WA, Allen KE (2009) Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg 21(1):44–57
Mao YZ, Tang S, Li S (2017) Comparison of the Nuss versus Ravitch procedure for pectus excavatum repair: an updated meta-analysis. J Pediatr Surg 52(10):1545–1552
Graves CE, Moyer J, Zobel MJ, Mora R, Smith D, O’Day M, Padilla BE (2019) Intraoperative intercostal nerve cryoablation during the Nuss procedure reduces length of stay and opioid requirement: a randomized clinical trial. J Pediatr Surg 54(11):2250–2256
Koh JC, Song Y, Kim SY, Park S, Ko SH, Han DW (2017) Postoperative pain and patient-controlled epidural analgesia-related adverse effects in young and elderly patients: a retrospective analysis of 2435 patients. J Pain Res 10:897–990
Stroud AM, Tulanont DD, Coates TE, Goodney PP, Croitoru DP (2014) Epidural analgesia versus intravenous patient-controlled analgesia following minimally invasive pectus excavatum repair: a systematic review and meta-analysis. J Pediatr Surg 49(5):798–806
St. Peter SD, Weesner KA, Weissend EE, Sharp SW, Valusek PA, Sharp RJ, Snyder CL, Holcomb GW, Ostlie DJ, (2012) Epidural vs patient-controlled analgesia for postoperative pain after pectus excavatum repair: a prospective, randomized trial. J Pediatr Surg 47(1):148–155
Lukosiene L, Rugyte DC, Macas A, Kalibatiene L, Malcius D, Barauskas V (2013) Postoperative pain management in pediatric patients undergoing minimally invasive repair of pectus excavatum: the role of intercostal block. J Pediatr Surg 48(12):2425–2430
Beltran R, Veneziano G, Bhalla T, Kenney B, Tumin D, Bissonnette B, Tobias JD (2017) Postoperative pain management in patients undergoing thoracoscopic repair of pectus excavatum: a retrospective analysis of opioid consumption and adverse effects in adolescents. Saudi J Anaesth 11(4):427
St Peter SD, Weesner KA, Sharp RJ, Sharp SW, Ostlie DJ, Holcomb GW (2008) Is epidural anesthesia truly the best pain management strategy after minimally invasive pectus excavatum repair? J Pediatr Surg 43(1):79–82
Litz CN, Farach SM, Fernandez AM, Elliott R, Dolan J, Nelson W, Walford NE, Snyder C, Jacobs JP, Amankwah EK, Danielson PD, Chandler NM (2017) Enhancing recovery after minimally invasive repair of pectus excavatum. Pediatr Surg Int 33(10):1123–1129
Man JY, Gurnaney HG, Dubow SR, DiMaggio TJ, Kroeplin GR, Adzick NS, Muhly WT (2017) A retrospective comparison of thoracic epidural infusion and multimodal analgesia protocol for pain management following the minimally invasive repair of pectus excavatum. Paediatr Anesth 27(12):1227–1234
Keller BA, Kabagambe SK, Becker JC, Chen YJ, Goodman LF, Clark-Wronski JM, Furukawa K, Stark RA, Rahm AL, Hirose S, Raff GW (2016) Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg 51(12):2033–2038
US Centers for Medicare & Medicaid Services (2020) Opioid oral morphine milligram equivalent (MME) conversion factors. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Oral-MME-CFs-vFeb-2018.pdf. Accessed 19 Jun 2020
Nuss D, Obermeyer RJ, Kelly RE (2016) Nuss bar procedure: past, present and future. Ann Cardiothorac Surg 5(5):422
Loftus PD, Elder CT, Russell KW, Spanos SP, Barnhart DC, Scaife ER, Skarda DE, Rollins MD, Meyers RL (2016) Paravertebral regional blocks decrease length of stay following surgery for pectus excavatum in children. J Pediatr Surg 51(1):149–153
Melnick GA, Fonkych K (2016) Hospital prices increase in California, especially among hospitals in the largest multi-hospital systems. Inquiry 53:0046958016651555
Weissler EH, Sanati-Mehrizy P, Massenburg B, Jenny H, Taub PJ, Midulla PS (2016) Abstract complications, length of stay, and economic burden among children undergoing pectus excavatum repair. Plast Reconstr Surg Glob Open 4(9):198–199
Lloyd J, Barnard J, Glynn C (1976) Cryoanalgesia: a new approach to pain relief. Lancet 308(7992):932–934
Katz J, Nelson W, Forest R, Bruce D (1980) Cryoanalgesia for post-thoracotomy pain. Lancet 315(8167):512–513
Roberts D, Pizzarelli G, Lepore V, Al-Khaja N, Belboul A, Dernevik L (1988) Reduction of post-thoracotomy pain by cryotherapy of intercostal nerves. Scand J Thorac Cardiovasc Surg 22(2):127–130
Papic JC, Finnell SME, Howenstein AM, Breckler F, Leys CM (2014) Postoperative opioid analgesic use after Nuss versus Ravitch pectus excavatum repair. J Pediatr Surg 49(6):919–923
Hall Burton DM, Boretsky KR (2014) A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth 24(5):516–520
Graves C, Idowu O, Lee S, Padilla B, Kim S (2017) Intraoperative cryoanalgesia for managing pain after the Nuss procedure. J Pediatr Surg 52(6):920–924
Erinjeri JP, Clark TW (2010) Cryoablation: mechanism of action and devices. J Vasc Interv Radiol 21(8 Suppl):S187–S191
Zobel MJ, Ewbank C, Mora R, Idowu O, Kim S, Padilla BE (2020) The incidence of neuropathic pain after intercostal cryoablation during the Nuss procedure. Pediatr Surg Int 36(3):317–324
Author information
Authors and Affiliations
Contributions
RLR, AGR, HYL, DBS, AHC, and RMS conducted background research, conducted the study, and co-wrote/edited the manuscript. RLR and RMS also conducted the statistical analysis and interpreted the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rettig, R.L., Rudikoff, A.G., Lo, H.Y.A. et al. Cryoablation is associated with shorter length of stay and reduced opioid use in pectus excavatum repair. Pediatr Surg Int 37, 67–75 (2021). https://doi.org/10.1007/s00383-020-04778-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04778-x