Abstract
Background
Currently, there are two well-established methods of bowel lengthening in patients with short bowel syndrome (SBS)-longitudinal intestinal lengthening and tailoring (LILT) and serial transverse enteroplasty (STEP) [1–4]. Both procedures may carry a high reported morbidity and mortality of 30.2% and 14.4%, respectively [5]. We report the outcomes of a novel technique: double barrel enteroplasty (DBE) for autologous intestinal reconstruction.
Methods
We performed a retrospective review of all ten patients who underwent DBE at our institution since 2011. All patients have SBS and were dependent on parenteral nutrition (PN) at the time of surgery. Etiologies were gastroschisis (n = 4), bowel atresia (n = 3), necrotising enterocolitis (n = 1), volvulus (n = 1), and near-total intestinal aganglionosis (n = 1). Patient survival, complications, and subsequent enteral autonomy were evaluated.
Results
All patients are alive with normal liver function. Five children achieved enteral autonomy, while the remaining are on weaning PN. There was no bleeding, anastomotic leak, perforation, infective complications, or intestinal necrosis. No patient has required a liver and/or intestinal transplant.
Conclusions
Double barrel enteroplasty is technically feasible and safe. It has similar efficacy and may have fewer complications when compared with other methods of autologous intestinal reconstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autologous intestinal reconstruction surgery has significantly improved the chance of intestinal autonomy in children with short bowel syndrome (SBS) [1]. Currently, the two most commonly used procedures of bowel lengthening in children are longitudinal intestinal lengthening and tailoring (LILT) [2] and serial transverse enteroplasty (STEP) [3, 4] pioneered by Bianchi and Kim, respectively. However, these can be high-risk procedures with not insignificant morbidity and mortality [5]. The concept of double barrel enteroplasty (DBE) originated in 2008 when author AS successfully treated an infant with total colonic duplication in Papua New Guinea during one of his humanitarian visits. A baby girl presented with a type II colonic duplication [6], but it was atypical in that only one end of the distal hemi-colon connected with her vagina and the other hemi-colon ended normally in the rectum. As it was essential to retain adequate bowel length for a baby in a developing country to survive, the duplicated double- barrel colon was retained and simply anastomosed end-to-end to the single lumen rectum after the duplicated distal segment of bowel to the vagina was resected. The baby thrived with no further problems. If two large bowel lumens in parallel can function normally, it stands to reason that two small bowel lumens can likewise do so. This provided the equipoise for the DBE technique. The double barrel method of bowel lengthening has theoretical advantages compared to the STEP and LILT, since there may be less disruption to the basic longitudinal continuity of the bowel and its mesentery with maintenance of the normal orientation of the bowel musculature, lymphatics, and innervation. With the original LILT technique when the two hemi-bowel loops are joined in series to achieve isoperistaltic orientation, there can be significant traction on the mesenteric vessels and nerves, particularly if the mesentery is short. This problem was first alluded to by Aigrain et al. [7] who described a circular orientation of the two hemi-bowel loops when they are joined to overcome this problem. Author AS has also encountered this problem in a case previously where the proximal loop of the two hemi-loops became obstructed due to the acute kinking of the bowel at the anastomosis as the mesentery at this point was short. Shah et al. [8] probably recognised this problem and did not use their institution’s preferred LILT technique, rather than the STEP, in their small bowel autologous reconstruction when the “mesenteric vasculature configuration of the dilated bowel” was unsuitable. With the STEP, as both the circular and longitudinal muscle fibres are divided at multiple sites, abnormal peristalsis may occur resulting in recurrent pathological re-dilation [9, 10].
Patients and methods
We performed a retrospective cohort review of all ten patients who underwent a DBE at our institution between 2011 and 2018. All patients had a diagnosis of SBS. The indication for performing this procedure was that: (1) All patients were dependent on PN at the time of surgery. (2) All patients had radiological evidence of dilated small bowel complicated by one or more of the following: (a) inability to grade up feeds and wean PN;(b) evidence of (partial) bowel obstruction despite imbrication of their dilated small bowel as described by de Lorimer and Harrison [11]; and (c) recurrent small bowel bacterial overgrowth (SBBO).
Written informed consent to both the DBE procedure and this report was provided by the parents of all the children. The study was approved by our hospital’s ethics committee. Data were collected retrospectively from patients’ medical records and from the pediatricians involved in the ongoing care of these children. Analysis included patient demographics, SBS etiology, small bowel length pre- and post-DBE, post-operative complications, and additional surgeries required. The percentage of remaining colon was determined as > 50% if the transverse colon was present. Clinical outcome data included PN dependence, anthropometric parameters, and cholestasis, defined as total bilirubin > 20 µmol/L. Weight and height were expressed as z-scores for age and sex.
Nutritional and medical management
Trophic feeds were started as early as possible after initial surgery and gradually advanced according to tolerance. Oral and/or nasogastric bolus feeding was used, or continuous feeding if failed to progress with bolus feeding. Breast milk was the preferred form of nutrition. If not available or not tolerated, generally lactose-free extensive hydrolysed formula was used during the first year of life and changed to a more age appropriated formula after that. Solids were normally introduced at the age of 4–6 months where possible.
Patients received PN overnight either in hospital and/or at home via an implanted central venous catheter. PN was administered by parents/carers after formal extensive training. PN composition and volumes were adjusted according to patients’ requirements, ensuring adequate hydration and growth. SMOF lipid (Fresenius Kabi, Bad Homburg, Germany) emulsion was the lipid of choice. PN solutions were routinely supplemented with fat- and water-soluble vitamins and trace elements. PN was gradually weaned as the enteral intake increased and was well tolerated, as long as patients maintained satisfactory growth.
Operative technique
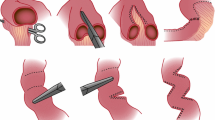
At laparotomy through the previous abdominal incision, all the adhesions were released. Small bowel length along its unstretched anti-mesenteric border was measured with a sterile flexible ruler from the duodenojejunal (DJ) flexure to the colon. The junction between the dilated proximal bowel and the normal calibre distal bowel, often at the site of the previous anastomosis, was transected. If present, the overhanging dilated “blind pouch” (Fig. 1) at the previous bowel anastomosis was removed. The arrangement of the vascular arcade in the mesentery of the dilated bowel was carefully appraised as previously described by Bianchi [2]. The alternating anterior and posterior mesenteric vessels that enter the bowel from either side of the midline to each half of the bowel were carefully separated to create a sufficient space to accept the anvil side of the gastrointestinal anastomosis stapler. When the stapler is applied, the vascularity to each half of the hemi-bowel loop must not be compromised. Extreme care was taken to avoid catching any blood vessel in the staple line when the jaws of the stapler were closed.
The lumen of each hemi-bowel was carefully scrutinised to ensure that the diameter of each lumen was the same before the stapler was fired with the formation of the two tubes in parallel from the dilated bowel. Either a GIA™ (Covidien autosuture 90 mm–2.5 cm) or a smaller laparoscopic version (Covidien Multifire Endo GIA™ autosuture 30–2.5 mm) stapler was used depending on the size of the mesenteric gap and the size of the bowel to be divided. This process was continued in a distal-to-proximal direction until relatively normal-sized bowel or the dilated duodenum was reached. If the proximal limit was the dilated duodenum, this was imbricated with non-absorbable Prolene suture to achieve a conical shape with reduced luminal calibre to ensure that the motility of this portion of the bowel was optimised. Where the proximal end was the slightly dilated jejunum but not wide enough to be partitioned, it was left alone or imbricated for about 5 cm (Fig. 2). As currently there is no normogram of what diameter of bowel is considered normal in a non-distended state after the contents are evacuated in children of various ages, the proximal point where the longitudinal division should end is subjected to intuitive assessment. With the children in this series, it is based on an estimate by two experienced pediatric surgeons. On most occasions, this is very close to the DJ flexure and ends where the availability of dividable mesentery ends. The diameter of the dilated bowel would mostly be about 5–6 cm in the older child and about 4 cm in a 2-year-old child. The thickness and in particular the floppiness of the bowel is taken into consideration in determining whether the bowel can and should be longitudinally divided. In extending the longitudinal division proximally, we err on the side of stopping where the bowel is just about less than twice the diameter of what we consider to be normal size bowel and imbricated the mildly dilated bowel as not to cause any possible narrowing and obstruction to the flow of the chyme.
Distally the double-barrelled bowel was joined either end-to-end onto the single lumen bowel if the discrepancy in size was not excessive. In doing this anastomosis, the common wall of the two half segments was carefully re-united and joined end-to-end to the distal bowel lumen with a single layer of inversion absorbable suture. If the size disparity was significant, one hemi-loop was joined end-to-end onto the distal bowel and the other hemi-loop was joined end-to-side onto the distal bowel just beyond the previous anastomosis (Fig. 2). The two hemi-bowel loops were left in parallel. This is the main distinction between the DBE and the LILT. We believe that this is important to prevent kinking of the bowel anastomosis when the mesentery is short between the two ends of the isoperiltaltic bowel loops in the standard LILT. Our method of anastomosis to restore bowel continuity also prevents any traction on the mesenteric blood vessels, lymphatics, and nerves therein. The anti-mesenteric borders of the two hemi-bowel loops were then loosely re-approximated with interrupted sutures to prevent exposure of the staples as they may aggravate adhesive bowel formation (Fig. 2). However, with our most recent infant (patient 10), it was noted that following this manoeuvre, the medial most end of the bowel (close to the staple line and the watershed area of the bowel) became somewhat ischemic looking and, therefore, these sutures were removed following which the vascularity returned to normal. The total length of small bowel is then measured along the anti-mesenteric border. Each limb of the DBE is measured separately and then added together.
Follow-up post-DBE
All patients were followed up by the surgical team and a gastroenterologist post-DBE, except for one overseas patient who was followed up by his local pediatrician. Clinical outcome data were collected around 3, 6, and 12 months after DBE and yearly after that until last available medical follow-up. PN dependency was determined as previously described by Lambe et al. [12] as the ratio between non-protein energy intake (NPEI)/resting energy expenditure (REE) calculated using the Schofield equation [13]. Children with NPEI/REE ratio > 120% were considered extremely dependent on PN, between 120 and 80% highly dependent, 80 and 40% mildly dependent, and < 40% on their way to weaning PN.
Results
Ten children (seven males) with SBS complicated by small bowel dilatation underwent DBE. Underlying diagnosis included intestinal atresia in three children, gastroschisis in four (including three children with vanishing gastroschisis), and the remaining due to necrotizing enterocolitis (NEC), midgut volvulus, and near-total intestinal aganglionosis, respectively. Median small bowel length at initial surgery was 34.5 cm (interquartile range, IQR 24.3–58.5). Nine children had their colon in continuity, but only two had a preserved ileocecal valve. (Table 1).
Surgical management and complications
The median age at the time of DBE was 21.2 months (IQR 9.4–27.4). Median small bowel length at the time of the procedure was 51 cm (IQR 45–92.8) and increased to 82.5 cm (IQR 70–122.3) following DBE, which represents a 23–100% increase in bowel length. (Table 2).
A unique procedure was required for the child with long-segment Hirschsprung’s disease. A 5 cm myotomy in the aganglionated bowel proximal to the stoma was performed. The distal 60 cm of aganglionated bowel was retained in the hope that it may provide some enteral absorption of nutrients. It was anastomosed end-to-side onto the myotomised aganglionated bowel just proximal to the stoma with the distal end of this segment matured as a stoma adjacent to where the myotomised aganglionated bowel was exteriorized as an end stoma (Fig. 3).
Three patients required additional surgery after DBE for recurrent vomiting. In two of these patients, the upper contrast study was unhelpful in delineating the problem as our radiologists were unable to follow the contrast agent separately in the two hemi-bowel loops. On upper endoscopy during laparotomy, when methylene blue was instilled into the duodenum, the dye could be seen egressing rapidly into both of the bowel lumens. There was no obvious preference for the dye to flow into one or the other lumen. The stricturoplasty was done by incising the narrow bridge of bowel longitudinally and closing it transversely.
None of the patients had bleeding complications in the early post-operative period. Two patients required subsequent upper and lower endoscopies to investigate the cause of their microcytic anaemia. In one, three small non-bleeding ulcers related to the metal staples were identified. No cause was identified in the other patient.
Median follow-up after DBE was 39 months (IQR 20.1–60.3). During this period, 6 patients achieved enteral autonomy and were transitioned to a normal diet. One of these patients, however, was recently restarted on PN support due to poor weight gain. All five patients currently on PN also receive an oral diet and/or enteral feeds. PN dependence decreased following DBE as shown by the NPEI/REE index which ranged from 88 to 140% pre-DBE to 48–75% at the last follow-up, while adequate growth was maintained (Fig. 4a). Patients’ growth parameters before and after DBE are shown in Fig. 4b. All patients reviewed are alive, with normal total bilirubin levels on most recent follow-up. To date, no patient has required an intestinal and/or liver transplant. For detailed information on individual patients, please see Supplementary table.
Nutritional outcomes and growth parameters of patients before and after DBE. a Individual levels of PN dependence determined by the ratio between non-protein energy intake (NPEI)/resting energy expenditure (REE) and expressed as a percentage. b Median weight-for-age and height-for-age z-scores and interquartile ranges at different time points
Discussion
With appropriate care by a dedicated multi-disciplinary bowel rehabilitation team, many children with SBS can now achieve enteral autonomy [14]. PN can mostly now be customised so that PN-related liver disease can mostly be avoided. However, some children with short dilated bowel still require autologous intestinal reconstruction to improve the gut motility, increase the absorptive capacity of the enterocytes, and prevent bacterial stasis with overgrowth and recurrent central line sepsis. Currently, there are two well-known methods of bowel lengthening, LILT, and STEP. Both procedures can be life-saving but both can also be problematic and fail to achieve their objectives [5]. The role of surgery in children with SBS should not just be a procedure merely to gain additional bowel length. There is a complex interaction between the absolute length of the bowel, the mucosal surface area available for absorption, the transit time, and effective peristalsis. The goal should be to optimise absorption by the available bowel with the least disruption to intestinal physiology. STEP and LILT theoretically increase transit time but if they are at the cost of ineffective peristalsis, increased risk of re-dilation, and therefore poorer absorption and greater risk of bacterial overgrowth, they are perhaps counterproductive. We believe optimal luminal calibre with unobstructed antegrade flow is more important and more effective. Although we cannot substantiate that the DBE is better than the LILT and STEP in achieving that, we believe that it may have a better chance.
Common sequelae of both LILT and STEP are re-dilation of the affected bowel which may or may not require re-operation [5, 9, 10]. Recurrent pathological re-dilation of the bowel post LILT or STEP is indeed not a universal occurrence. Frongia et al. [5] reported the incidence to be around 39% (8–100%) after LILT and 49% (30–67%) after STEP. Pathological re-dilation, rather than the normal degree of dilation from adaptation, can be a major problem. It is linked to failure to achieve enteral autonomy in affected children [1, 5, 14]. The underlying causes for the pathological re-dilation of the short bowel are unknown, but are likely to be multifactorial. We suspect a disturbance in the motility of the bowel due either to subtle mechanical obstruction as alluded to above with the LILT and/or disruption of the muscle arrangement of the bowel with the STEP may be contributing factors which are prevented with the DBE technique.
All shortened bowel will undergo some compensatory dilation due to the adaption process. We are hopeful that the DBE orientation of the bowel may be less likely to undergo pathological re-dilation as with this modification there is less disruption to the overall bowel anatomy given that the orientation of the bowel musculature, lymphatics, and nerves are less disrupted compared to the LILT and STEP. With the DBE technique, no child so far has needed a re-operation because of pathological re-dilation of the instrumented bowel. This indeed may be due to the short follow-up of our patients. We recognise that late re-dilation after LILT has also been reported [15] and our children remain closely monitored for this. With the original LILT technique described by Bianchi [2], where the two hemi-bowel loops are joined in series to achieve isoperistaltic orientation there can be significant traction on the mesenteric vessels, lymphatics, and nerves as already alluded to above. In addition, with the DBE as the two hemi-bowel loops are side by side along their entire length, they may buffer each other from internal distending forces resulting in pathological re-dilation. We have had one child who required a re-operation, because our imbrication of the duodenum failed. At re-operation, the previous DBE bowel was not pathologically dilated, although the duodenum and a short segment of jejunum cephalad to the previous instrumented bowel were. Longer term follow-up and a larger number of patients will help to clarify this point of supposition.
Amongst a number of possible mechanisms that may be responsible for the impaired absorption of nutrients by the enterocytes in the dilated gut, one would be bacterial stasis with overgrowth, resulting in an inflammatory response and villous atrophy in the bowel [16]. The second would be an inability of the enterocytes to come in contact with the nutrients in the dilated bowel due to the increased distance between the enterocytes and the nutrients in the central column of the succus entericus in the dilated bowel. Both of these problems are ameliorated by the DBE as with any successful LILT or STEP. Normal motility is a very important factor in the success of any intestinal reconstruction procedure. Poor motility can be due to either functional or mechanical issues. With the DBE, the peristalsis following the longitudinally stapled bowel division does not appear to be hindered. Independent normal peristalsis by each limb of the hemi-bowel loop is clearly visible at the time of surgery and at the subsequent laparotomies in the three patients who required it. The absence of any kinking of the bowel lumen or prevention of traction nerve injury or subtle mechanical obstruction to the bowel may be contributing factors to the maintenance of normal peristalsis after DBE. Could these be the reasons why obstruction/stricture after LILT tends to be more prevalent during the first post-operative year as noted by Frongia et al. [5]?
With patients 1, 3, and 6, who appeared to have sufficient native bowel lengths to allow them to achieve enteral autonomy without autologous intestinal lengthening, one may argue that the DBE may be unnecessary. However, as both patients 1 and 6 had already failed an imbrication procedure, an observation which has previously been noted [17, 18] and that Kang et al. [9] as well as Kim et al. [4] had previously shown that bowel length of greater than 100 cm per se cannot reliably in all cases predict achievement of enteral autonomy, we treated these three patients with DBE to optimise the chance of enteral autonomy as soon as possible to avoid problems associated with prolonged PN dependence. This is particularly so with patient 1 as there is no home PN available where her family resides.
The length of the dilated bowel that can be partitioned is not limited by the foreshortened mesentery in contrast to Shah et al.’s experience [8]. With the DBE, as long as the blood supply in the mesentery to each hemi-loop can be preserved and not injured by the stapler, the bowel can be safely divided into two throughout the length of the dilated bowel as seen in Patient 9. Frongia et al. [5] in their review suggested that the Bianchi technique is best suited for dilated bowel lengths of 20–40 cm as “if LILT was performed in shorter remnant intestinal segments failure and mortality rates were clearly higher”. We have had no bowel necrosis, anastomotic leaks, or bleeding as reported by others [5, 19, 20].
One aspect of bowel architecture that has been poorly studied to date is the lymphatic channels in the shortened gut. The whole-mount confocal images of the lymphatic channels in the adapting shortened gut very elegantly displayed by Onufer et al. [21] would, theoretically, be interfered with the least with the DBE technique. This may have important implications for absorption and transport of nutrients as well as gut motility given that any edematous bowel is not likely to function optimally.
Total colonic Hirschsprung disease with extensive small bowel involvement is a well-recognised cause of SBS in children. The DBE procedure for our 9th patient at this early stage appears to have major benefits for his overall wellbeing. It has not only ameliorated the management of his fluids and electrolyte problems but has also allowed him to tolerate his oral intake with a decreased reliance of his PN.
The absence of the ICV in children with SBS has been considered to be an important factor by Mutanen et al. [22] recently to be a possible factor responsible for the re-dilation of the bowel and failure to achieve enteral autonomy post-STEP. In our small series, three of our patients without an ICV have achieved enteral autonomy post-DBE, an observation is of uncertain significance.
Patient 10 underwent the DBE at an early age of 3 months. We did not consider early age to be a contraindication to autologous intestinal reconstruction as a number of reports [23,24,25] have shown this to be beneficial. The exact timing of autologous reconstruction is controversial [5, 17, 26] as there is currently no study demonstrating delaying the surgery offers a better outcome. The timing of the surgical intervention needs to be individualized [17]. Wood et al. [27] have shown that in the 8 children who underwent lengthening surgery at < 365 days compared to the 6 children who had their procedure done > 365 days achieved enteral autonomy much earlier at 17 months compared to 59 months in the late group. The earlier patients also required significantly less number of central venous catheters.
There are a number of limitations to this study and must be carefully considered when interpreting the results. This is a retrospective study; the number of patients is small; the etiology of the children’s SBS is heterogenous; delayed bowel re-dilation remains a significant concern and careful longer term follow-up will be necessary to monitor for this. However, should re-dilation occur, it should be amenable to a size reduction with hopefully a satisfactory outcome [28]. There is also an assumption that luminal contents of the bowel would flow more or less equally down both lumens of the separated bowel. Unfortunately, this is very difficult to demonstrate on routine radiological examination as we have found in two of our patients. It is also possible that one lumen of the hemi-loop may become either obstructed or become the dominant channel and results in recurrent bowel obstruction. Despite these limitations, all of our patients to date have benefited from the procedure, ranging from normalisation of their liver function tests to a lesser requirement for PN as well as complete weaning off PN with satisfactory growth. In conclusion, we suggest the DBE, a modification of the Bianchi LILT technique, may be a useful alternative for autologous intestinal reconstruction to enhance intestinal adaptation in children with SBS of various etiology. The technique has been shown to be feasible and safe in our small series of patients on short-term follow-up.
References
Sudan D, Thompson J, Botha J (2007) Comparison of intestinal lengthening procedures for patients with short bowel syndrome. Ann Surg 246:593–604
Bianchi A (1980) Intestinal loop lengthening—a technique for increasing small intestinal length. J Pediatr Surg 15(2):145–151
Kim HB, Fauza D, Gasza J et al (2003) Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg 38(3):425–429
Kim HB, LeePW GJ et al (2003) Serial transverse enteroplasty for short bowel syndrome: a case report. J Pediatr Surg 38(6):881–885
Frongia G, Kessler M, Weih S et al (2013) Comparison of LILT and STEP procedures in children with short bowel syndrome—a systematic review of the literature. J Pediatr Surg 48:1794–1805
Kottra JJ, Dodds WJ (1971) Duplication of the large bowel. Am J Roentgenol 113:310
Aigrain A, Cornet D, Cezard JP (1985) Longitudinal division of small intestine: a surgical possibility for children with very short bowel syndrome. Z Kinderchir 40:233–236
Shah AA, Petrosyan M, Franklin A et al (2019) Autologous intestinal reconstruction a single institution study of serial transverse enteroplasty (STEP) and the longitudinal intestinal lengthening and tailoring (LILT). Pediatr Surg Int. https://doi.org/10.1007/S00383-019-04468-3
Kang KH-J, Gutierrez I, Zurakowski D et al (2012) Bowel re-dilation following serial transverse enteroplasty (STEP). Pediatr Surg Int 28:1189–1193
Miyasaka EA, Brown PI, Teitelbaum DH (2011) Redilation of bowel after intestinal lengthening procedures-an indicator for poor outcome. J Pediatr Surg 46:145–149
de Lorimier AA, Harrison MR (1983) Intestinal plication in the treatment of atresia. J Pediatr Surg 18(6):734–737
Lambe C, Poisson C, Rocha A et al (2017) The NPEI/REE Ratio: a new dependency index in paediatric parenteral nutrition? Transplantation 101(6S2):S77
Schofield WN (1985) Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39(Suppl 1):5–41
Barrett M, Demehri FR, Ives GC et al (2017) Taking a STEP back: assessing the outcomes of multiple STEP procedures. J Pediatr Surg 52:69–73
Höllwarth ME (2017) Surgical strategies in short bowel syndrome. Pediatr Surg Int 33:413–419
Goulet O, Nader EA, Pigneur B et al (2019) Short bowel syndrome as the leading cause of intestinal failure in early life: some insights into the management. PGHN 22(4):303–329
Sommovilla J, Warner BW (2014) Surgical options to enhance intestinal function in patients with short bowel syndrome. Curr Opin Pediatr 26:350–355
Stringer MD, Puntis JW (1995) Short bowel syndrome. Arch Dis Child 73:170–173
Fitzgerald K, Muto M, Belza C et al (2019) The evolution of the serial transverse enteroplasty for pediatric short bowel syndrome at a single institution. J Pediatr Surg. https://doi.org/10.1016/j.jpedsurg.2019.01.051
King B, Carlson G, Khalil BA et al (2013) Intestinal bowel lengthening in children with short bowel syndrome: systemic review of the bianchi and STEP procedures. World J Surg 37:694–704. https://doi.org/10.1007/S00268-012-1879-3
Onufer EJ, Czepielewski R, Seiler KM et al (2019) Lymphatic network remodeling after small bowel resection. J Pediatr Surg 54(6):1239–1244
Mutanen A, Barrett M, Feng Y et al (2019) Short bowel mucosal morphology, proliferation and inflammation at first and repeat STEP procedures. J Pediatr Surg 54:511–516
Lobos PA, Calello SEM, Busoni VB et al (2016) Neonatal serial transverse enteroplasty (STEP): case report. Transpl Proc 48(2):528–531 (PMID: 27109993)
Bhalla VK, Pipkin WL, Hatley R et al (2013) The use of multiple serial transverse enteroplasty (STEP) procedures for the management of intestinal atresia and short bowel syndrome. Am Surg 79:826–829
Bianchi A (1984) Intestinal lengthening: an experimental and clinical review. J R Soc Med Suppl 3(77):35–41
Garnett GM, Kang KH, Jaksic T et al (2014) First STEPs: serial transverse enteroplasty as a primary procedure in neonates with congenital short bowel. J Pediatr Surg 49:104–108
Wood SJ, Khalil B, Fusaro F et al (2013) Early structured surgical management plan for neonates with short bowel syndrome may improve outcomes. World J Surg 37(7):1714–1717
Dewberry LC, Hilton SA, Vuille-Dit-Bille R (2020) Is tapering enteroplasty an alternative to resection of dilated bowel in small intestinal atresia. J of Surg Res 246:1–5 (PMID: 31541708)
Acknowledgements
We would like to thank Dr. Yann Polfrit, Pediatrician, Centre Hospitalier Territorial Gaston-Bourret, Noumea, New Caledonia and Dr. Scott Nightingale from the Department of Paediatric Gastroenterology, John Hunter Hospital in Newcastle, NSW, Australia, for follow-up care of patients 1, 2, and 5, respectively. Dr, Gordon Thomas provided the illustrations.
Funding
This research did not receive any grants from any public or private funding agencies.
Author information
Authors and Affiliations
Contributions
All authors participated in the data acquisition, in patient care, and critical revision of the manuscript. The first draft of the manuscript was written in full by Albert Shun and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shun, A., Thomas, G., Puppi, J. et al. Double barrel enteroplasty for the management of short bowel syndrome in children. Pediatr Surg Int 37, 169–177 (2021). https://doi.org/10.1007/s00383-020-04767-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04767-0