Abstract
Introduction
Oesophageal atresia ± tracheoesophageal fistula (EA/TEF) associated with congenital heart disease (CHD) carries a worse prognosis than EA/TEF alone. Though the Spitz classification takes major CHD into account, there are no data regarding survival with the specific combination of EA/TEF and Tetralogy of Fallot (TOF). With advances in postnatal care, we hypothesised that, survival is improving in these complex patients. This study reports morbidity and mortality outcomes of newborns with oesophageal atresia and TOF cardiac malformations
Methods
All patients with EA/TEF and TOF treated at Alder Hey Children’s Hospital between the years 2000–2020, were identified. Data sets regarding gestation, birth weight, associated anomalies, operative intervention, morbidity, and mortality were analysed.
Results
Of a total of 350, EA/TEF patients 9 (2.6%) cases had EA/TEF associated with TOF (M:F 4:5). The median gestational age was 35/40 (range 28–41 weeks) with a median birth weight of 1790 g (range 1060–3350 g). Overall survival was 56% (5/9 cases) and all survivors remain under follow up (range 37–4458 days). Surgical strategies for managing EA/TEF with Fallot’s tetralogy included 6/9 primary repairs and 3/9 cases with TEF ligation only (+ gastrostomy ± oesophagostomy).
Conclusions
This study reports outcome data from one of the largest series of EA TEF patients with Fallot’s tetralogy. Whilst outcomes may be challenging for this unique patient cohort, survival metrics provide important prognostic information that can be widely shared with health care teams and parents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oesophageal atresia (EA) is a rare congenital anomaly, with an incidence of 0.3 per 1000 live births [1] and there is a well-recognized association of newborns also having congenital heart disease (CHD). The Spitz classification system (1994) provides a robust prognostic scoring tool that shows a survival correlation with infant birth weight and presence or absence of congenital heart disease [2, 3]. Survival for isolated EA is almost 98%; however, when EA TEF is associated with a major cardiac anomaly and low birth weight, less than 50% of patients experience hospital discharge [3]. Outcomes for Tetralogy of Fallot (TOF) have significantly improved over the past 20 years, with notable advances in cardiac surgery, and such that survival beyond adolescence is reported in around 80% of cases [4]. To the best of our knowledge, few (if any) large studies, have analysed clinical outcome data of EA/TEF newborns with TOF. The aim of this study, sought to reappraise outcome metrics in a modern era of care for this high-risk patient cohort, managed at a high-volume centre specialising in the multidisciplinary care of newborns with complex oesophageal atresia anomalies.
Methods
Medical records of all newborns with a diagnosis of EA, TEF and TOF presenting to Alder Hey Children’s Hospital, Liverpool, UK during a 20-year time period (2000–2020) were analysed. Information from clinical coding records, cross-checked with archived notes, clinic letters and operation records, ensured strict accuracy of disease phenotype(s). For the nine cases we identified, the following data were collected: gender, antenatal/postnatal imaging, pregnancy and delivery complication(s), gestational age, birth weight, date, age and cause of death (where available), Gross EA TEF classification, VACTERL associations and operative procedures.
Statistical analysis used GraphPad Prism© software (version 7.02, GraphPad Software Inc.). All data were tested for normality using the Shapiro–Wilk test. Unpaired t tests with Welch’s correction (assuming data do not have equal standard deviations) were deployed for comparison of means between survivors and non-survivors. Difference(s) in gender distribution between those who survived and the non-survival cases were analysed with Fisher’s exact test. Correlation analysis between age at death and birthweight or week(s) of gestation, was performed with non-parametric Mann–Whitney U tests. Statistical significance p < 0.05.
Results
Three hundred and fifty cases of EA/TEF were identified during the 20-year study period, 9 cases (M:F 4:5) having EA/TEF with TOF (Table 1). Tetralogy of Fallot was suspected antenatally in all patients. EA was also suspected in 4/9 pregnancies (44%). Gestational age at birth ranged between 28 and 41 weeks. Median birth weight was 1790 g (range 1060–3350 g). Overall survival of EA TEF with Fallot’s tetralogy was 56%. The majority of cases (n = 7) were of Gross type C EA (75%), with two cases having Gross type D EA (25%) [5].
Six cases had successful primary oesophageal anastomosis and ligation of TEF, with five patients here surviving (83%). A single patient required a defunctioning colostomy for an anorectal malformation.
Three cases had TEF fistula ligation only, with the formation of a feeding gastrostomy; one of whom also required a cervical oesophagostomy and duodenojejunostomy for congenital duodenal atresia. This complex patient had associated chromosomal duplication(s). No single patient here, who had TEF fistula ligation and feeding gastrostomy, survived long enough to have staged oesophageal repair. The median age at death was 121 days (range 10–159 days).
For the four non-surviving patients, causes of death included: (a) sepsis, (b) withdrawal of active medical care due to ventilator dependence, (c) global ischaemic hypoxic brain injury following a prolonged cardiac arrest after stenting of the right ventricular outflow tract and (d) pulmonary haemorrhage. All four patients were born prematurely, before 35 weeks gestation. There was a statistically significant correlation between prematurity and death (p = 0.0159) (Fig. 1). All patients who died had significantly lower birthweights compared with survivors (p = 0.0317) (Fig. 2). There were no gender differences comparing those who survived and non-survivors (p = 0.486).
Discussion
The results of this study, clearly demonstrate the fragility of EA TEF newborns with Tetralogy of Fallot, with a recorded survival rate of only 56% compared to > 95% survival noted in babies without major CHD [2].
The prevalence of EA TEF associated with CHD varies widely and is reported between some 15–70% of cases [1, 6]. To date, there are few (if any) published studies that specifically address the prognosis of EA TEF with TOF. EA TEF associated with TOF occurs in some 0.9–4% of all EA cases recorded in the literature [1, 7, 8]. At our specialist centre, with a large EA TEF patient cohort, the incidence rate was 2.6%.
The first successful EA TEF repair in a patient with TOF was reported in 1996, and at 1 year follow up, this infant was alive and well [9]. However, crucially, follow up beyond 1 year of age was not subsequently reported. Leonard et al. reported 26 cases of babies born with EA TEF and CHD. Two patients had the combination of EA TEF with TOF, both of whom died, one at 236 days from catastrophic oesophageal haemorrhage and the second child, during a cardiac surgery operation to repair the TOF at 455 days [1]. Stoll et al. documented five cases of EA TEF with TOF, but did not specifically analyse or report the survival outcomes for these patients [7]. Puri et al. described the largest series of patients with EA TEF and duct dependant heart disease (n = 124), strikingly, some 37 cases here had EA TEF with TOF [10]. They found mortality to be notably higher in EA TEF cases with duct dependent CHD vs CHD alone (22.1% vs 8.4%) but again did not specifically report the mortality data for EA TEF with TOF. This study additionally confirms our data findings that newborns with the EA/TEF/TOF phenotype are more likely to be born prematurely than babies with EA TEF and CHD disease alone. Another study, reporting on some 53 children with EA TEF (25 of whom had CHD), noted 100% survival in those without CHD; in EA TEF with non-duct dependant, CHD survival was 94.7% and in those babies with EA TEF and duct dependant CHD only 17% were alive. The authors here, unfortunately, do not define or report their EA TEF TOF cohort patient outcomes [11]. Categorising all patients with CHD together to offer survival prognosis scoring for EA TEF with TOF, lacks an evidence base. We submit that TOF represents a severe critical CHD phenotype in EA TEF patients linked with almost 50% mortality compared to any other forms of CHD.
Three significant findings in our study are worthy of note: (a) no survival in babies born < 35 weeks gestation, (b) no survival at birthweight < 1200 g, and (c) no survival in index cases where primary oesophageal anastomosis and TEF ligation was not achievable.
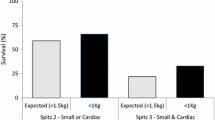
We found it unhelpful to evaluate our EA TEF and TOF phenotype using the currently available prognostic scoring systems (notably Spitz [2], Waterston [12] and Montreal classifications [13]) (Table 2). Considering Spitz classification, six of our patients would be defined as Spitz Group II, with a predicted survival of 59%; our study survival for this group was 83%. Three patients in our series would also have been categorised as Spitz Group III, with a predicted survival of 22%; in this group, we had zero survival. We, therefore, suggest that patients with critical duct-dependent CHD and EA TEF, cannot be readily offered accurate survival prognostic scoring using such methods (Table 3).
Recommendations for termination of pregnancy or palliation postnatally may be practiced, based on the current survival outcome data for EA TEF patients with Tetralogy of Fallot. It should be noted, however, that the survival for babies born with EA TEF and CHD, have substantially improved almost sevenfold over recent decades [1, 8]. Appreciating that EA TEF TOF patients are a very high-risk group is acknowledged, though we would counsel, from our experience herein reported, that high volume centres with MDT services managing a complex EA TEF case load may achieve perhaps better outcomes.
Conclusion
In summary, this study has clearly shown that better survival rates (56%) than hitherto reported, can be achieved in patients born with EA TEF and TOF; outcomes are not always dismal. Prematurity, low birthweight and failure of primary anastomosis are all contributory risk factors for mortality. In these complex clinical scenarios, it is vital to share this knowledge to aid counselling of parents, clinical decision making and the development of care pathways. We emphasise that multidisciplinary teamwork amongst health care professionals is crucial in the planning and delivery of care with such complex cases.
References
Leonard H et al (2001) The influence of congenital heart disease on survival of infants with oesophageal atresia. Arch Dis Child Fetal Neonatal Ed 85(3):F204–F206
Spitz L et al (1990s) Oesophageal atresia: at-risk groups for the 1990s. J Pediatr Surg 29(6):723–725
Spitz L (2007) Oesophageal atresia. Orphanet J Rare Dis 2:24
Tennant PW et al (2010) 20-year survival of children born with congenital anomalies: a population-based study. Lancet 375(9715):649–656
Gross RE (1953) The surgery of infancy and childhood. Its principles and techniques. W.D Saunders Co, Philadelphia
Sulkowski JP et al (2014) Morbidity and mortality in patients with esophageal atresia. Surgery 156(2):483–491
Stoll C et al (2017) Associated anomalies in cases with esophageal atresia. Am J Med Genet Part A 173(8):2139–2157
David TJ, O'Callaghan SE (1974) Cardiovascular malformations and oesophageal atresia. Br Heart J 36(6):559–565
Webber EM, Gillis DA, Ross DB (1996) Tetralogy of Fallot with total anomalous pulmonary venous drainage and esophageal atresia: complete correction in infancy. Ann Thorac Surg 62(2):571–573
Puri K et al (2018) Characteristics and outcomes of children with ductal-dependent congenital heart disease and esophageal atresia/tracheoesophageal fistula: a multi-institutional analysis. Surgery 163(4):847–853
Diaz LK et al (2005) Tracheoesophageal fistula and associated congenital heart disease: implications for anesthetic management and survival. Paediatr Anaesth 15(10):862–869
Waterston DJ, Carter RB, Aberdeen E (1962) Oesophageal atresia: tracheo-oesophageal fistula: a study of survival in 218 infants. Lancet 1(7234):819–822
Poenaru D et al (1993) A new prognostic classification for esophageal atresia. Surgery 113(4):426–432
Funding
This study has received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kathryn O’Shea declares that she has no conflict of interest. Megan Griffith declares that she has no conflict of interest. Alan King Lun Liu declares that he has no conflict of interest. Paul Losty declares that he has no conflict of interest. Matthew Jones declares that he has no conflict of interest. Joanne Minford declares that she has no conflict of interest. Fiona Murphy declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Shea, K.M., Griffiths, M.L., King, K.L. et al. Esophageal atresia and tracheoesophageal fistula associated with tetralogy of Fallot: a review of mortality. Pediatr Surg Int 36, 1243–1247 (2020). https://doi.org/10.1007/s00383-020-04732-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04732-x