Abstract
Purpose
Recently, plastic closure of abdominal defect in infants with gastroschisis has been used. Timing of gastroschisis closure can be mainly divided into two groups: primary closure and delayed closure after silo forming. Safety and usefulness of plastic closure in gastroschisis remains unclear. We aimed to evaluate the current evidence for plastic closure in infants with gastroschisis.
Methods
The analysis was done for primary closure as well as closure after silo. Outcomes were mortality, wound infection, duration of ventilation, time to feeding, and length of hospital stay (LOS). The quality of evidence was summarized using the GRADE approach.
Results
In the “primary” group, there was no significant difference in mortality, time to feeding initiation and LOS. In the “silo” group, wound infection was significantly lower in plastic closure (Odds ratio 0.24, 95%CI 0.09–0.69, p = 0.008). Duration of ventilation, time to feeding initiation and LOS were significantly shorter after plastic closure (Ventilation; mean difference (MD) − 5.76, p = 0.03. Feeding initiation; MD − 9.42, p < 0.0001. LOS; MD − 14.06, p = 0.002). Quality of evidence was very low for all outcomes.
Conclusions
Current results suggest that plastic closure may be beneficial for infants with gastroschisis requiring silo formation. However, this evidence is suboptimal and further studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroschisis is a congenital disease characterized by a defect of the abdominal wall and herniation of viscera through this defect [1, 2]. Definitive treatment of gastroschsis involves closure of abdominal wall defect. Traditionally, neonates with gastroschisis undergo either primary or staged reduction of herniated viscera, followed by surgical suture closure. In 2004, Sandler et al. reported plastic “sutureless” closure of abdominal wall defect using a synthetic wound dressing [3]. Since then, plastic (or sutureless) closure has been frequently used.
Although plastic closure can be performed without anesthesia at bedside, its safety and usefulness have not been established. A recent systematic review suggested that plastic closure has improved outcomes compared to traditional suture closure [4]. However, outcomes of gastroschisis can be affected by several factors with “timing of closure” being one of the most predominant factors. Neonates with gastroschisis can undergo primary closure of the abdominal defect or staged closure after silo formation [1]. Staged closure is chosen when primary closure is impossible due to edematous or thickened herniated bowel loop. It can take 7–10 days for the space in the abdominal cavity to enlarge and the intestinal loops being reduce into the abdomen without creating an abdominal compartment syndrome. Recent systematic reviews reported that primary closure had shorter duration of recovery, including duration of ventilation, time to full feeding and length of hospital stay [5, 6]. Therefore, the outcomes of gastroschisis closure should be evaluated for primary and staged closure. To our knowledge, there is no systematic review comparing plastic closure with suture closure for primary or staged repair of gastroschisis abdominal defect. Therefore, the aim of this study is to evaluate the current evidence, assessing the effectiveness and usefulness of plastic closure focusing on primary closure (primary) and staged closure of gastroschisis after silo formation (silo).
Materials and methods
This systematic review and meta-analysis was performed following the Cochrane Handbook for Systematic Reviews of Intervention and the Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) [7, 8]. The protocol of the systematic review was registered on the PROSPERO online database (PROSPERO 2017: CRD42018086513) on February 11, 2018 [9]. We searched MEDLINE and EMBASE using the combination of following terms: “gastroschisis”, “plastic”, “sutureless”, “ward”, and “bedside”. In addition, a manual search of the references of retrieved articles was performed. The last search was performed on May 10, 2018. We planned to include all published randomized controlled trials (RCTs) and observational studies. Mortality, complications, duration of ventilation, time to initiation of feeding, time to full feeding, and length of hospital (LOS) were the outcomes for this meta-analysis. We included all studies comparing the above outcomes in infants who underwent plastic closure or suture closure for gastroschisis for either primary or staged repair after silo formation.
Two reviewers (HM and SS) independently screened all retrieved abstracts with a low threshold for selecting studies for full-text review. There was no language restriction. Full texts were then independently reviewed to identify the included studies. In this step, we extracted the following data from each article: first author and year of publication, study design, country, year of study, sample size, type of gastroschisis, gestational age, birth weight, associated anomaly, and outcomes. Disagreements regarding inclusion were resolved through a discussion between reviewers, reaching consensus at each stage of the screening process.
We performed the meta-analysis using Review manager 5.3. We estimated statistical significance using a two-sided p-value of 0.05. Effect sizes were calculated and presented as pooled odds ratio (OR) along with a 95% confidence interval (CI) for dichotomous data and mean difference (MD) for the analysis of continuous data. A random-effects model was implemented using the Inverse Variance method.
The Grading of Recommendations and Assessment, Development and Evaluation (GRADE) system was used assessing the quality of the evidence [10,11,12,13,14,15,16,17]. Quality of evidence was rated as ‘high,’ ‘moderate,’ ‘low’ and ‘very low’ for each outcome. The initial quality of evidence for observational studies was considered low. The quality of evidence was then rated down in the presence of risk of bias, inconsistency, indirectness, imprecision and publication bias. For the assessment of the risk of bias in observational studies, we used the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [18]. The following domains were assessed for each outcome: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result. Each domain was scored as ‘low,’ ‘moderate,’ ‘serious,’ and ‘critical’ risk of bias and consecutively the overall risk of bias was scored. Heterogeneity was determined through the assessment of inconsistency using I2 statistics. To this extend, the I2 value of 0–40%, 30–60%, 50–90% and 75–100% were considered as ‘low,’ ‘moderate,’ ‘substantial,’ and ‘considerable’ heterogeneity, respectively. Imprecision was assessed using optimal information size (OIS), which was based on 25% relative risk reduction, 0.05 of α error and 0.20 of β error for dichotomous data. For continuous data, imprecision was assessed OIS based on 0.05 of α error, 0.20 of β error, 3.0 of Δ and 5.0 of standard deviation [19]. We planned to assess publication bias using funnel plots if ten or more studies were available. The quality of evidence was upgraded in the presence of large magnitude of effects, dose–response gradient and plausible confounders. Large magnitude of effect was present if the relative risk (RR) was greater than 2 or less than 0.5. We summarized the results of the meta-analyses and the assessment of quality of evidence for each outcome using GRADEpro GDT [20].
Results
Included studies/study selection
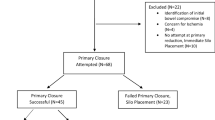
We identified 201 articles after removing duplicates. 146 articles were excluded during title and abstract screening. Full text screening was performed and no RCT was identified. Six retrospective cohort studies were selected for meta-analysis (Fig. 1) [21,22,23,24,25,26]. Four out of six studies compared plastic closure with suture closure for primary closure [21,22,23,24]. Four studies compared plastic closure with suture closure for the closure after silo formation [22, 24,25,26]. As no RCT was found, the meta-analysis was performed for observational studies. The characteristics of included studies are shown in Tables 1 and 2.
Mortality
Three studies in the ‘primary’ group and 3 studies in the ‘silo’ group reported mortality. In the ‘primary’ group, mortality rate was 1.7% (1/58) in plastic closure and 4.3% (2/47) in suture closure (Fig. 2a). There was no significant difference between two methods (OR 0.49, 95% CI 0.05–4.63, p = 0.53, I2 = 0%). In the ‘silo’ group, mortality was 2.4% (1/41) in plastic closure and 1.7% (1/59) in suture closure (Fig. 2b). There was no significant difference between two methods (OR 1.39, 95% CI 0.14–13.89, p = 0.78, I2 = 0%).
Wound infection
In the ‘silo’ group, wound infection was the single complication data could be extracted. The incidence of wound infection was 17.6% (6/34) in plastic closure and 37.2% (35/94) in suture closure (Fig. 3). Wound infection was significantly fewer in plastic closure compared to suture closure (OR 0.24, 95% CI 0.09–0.69, p = 0.008, I2 = 0%).
Duration of ventilation
Two studies reported duration of ventilation for the ‘silo’ group. The duration of ventilation was significantly shorter in plastic closure compared to suture closure for the ‘silo’ group (MD − 5.76. 95% CI − 10.93–0.60, p = 0.03, I2 = 0%, Fig. 4).
Feeding
Two studies in the ‘primary’ group and two studies in the ‘silo’ group reported time to initiation of enteral feeding (Fig. 5). In the ‘primary’ group, there was no significant difference between the two closure methods (MD − 3.14, 95% CI − 7.31 to 10.3, p = 0.14, I2 = 0%). In the silo group, time to initiation of the feeding was significantly shorter in plastic closure compared to suture closure (MD − 9.42, 95% CI − 13.68–5.16, p < 0.00001, I2 = 0%).
Two studies in the ‘silo’ group reported time to full feeding (Fig. 6). There was no significant difference in time to full feeding between two closure methods (MD − 6.78, 95% CI − 17.03 to 3.46, p = 0.19, I2 = 24%).
Length of hospital stay
Two studies in the ‘primary’ group and three studies in the ‘silo’ group reported LOS (Fig. 7). In the ‘primary’ group, there was no significant difference in LOS between two closure methods (MD − 6.85, 95% CI − 21.04 to 7.34, p = 0.34, I2 = 0%). In the ‘silo’ group, LOS was significantly shorted in plastic closure compared to suture closure (MD − 14.06, 95% CI − 22.86 to 5.26, p = 0.002, I2 = 0%).
Quality assessment
Evidence tables for GRADE assessment are shown in Tables 3 and 4. All outcomes for both the ‘primary’ and ‘silo’ group had a serious risk of bias according to ROBINS-I (Tables 5, 6). Inconsistency was not considered to be serious as the heterogeneity was low. Indirectness was also assessed as not serious. In all outcomes, our results did not meet OIS. Therefore, imprecision was considered serious. As there were only four included studies, we did not perform funnel plot analysis. There was no evidence to support publication bias. Because of a serious risk of bias and imprecision, we rated down the quality of the evidence for all outcomes. Overall, the quality of evidence was considered “very low” for all outcomes.
Discussion
In this review, we revealed that plastic closure compared to suture closure has less wound infection, shorter duration of ventilation, shorter time to initiation of feeding and shorter length of hospital stay for the patients requiring a closure using silo. In contrast, there was no significant difference between study groups in any of the evaluated outcomes such as mortality, time to initiation of enteral feeding and length of hospital stay. Because of a serious risk of bias and imprecision the quality of current evidence is considered very low for all outcomes.
Youssef et al. performed a systematic review comparing flap (plastic) closure with suture closure which indicates that flap closure had less wound infection, while increasing the risk of umbilical hernia [4]. These authors also reported that, while statistically not significant, flap closure had tendency to shorten duration of total parenteral nutrition (TPN), ventilation and time to initiation of feeding. Although these findings are important to understand the usefulness of plastic closure, the authors did not distinguish between primary closure and closure after silo formation. It has been reported that in gastroschisis staged closure resulted in longer hospital stay and longer time to reach full feeding [6]. Previous systematic review comparing plastic closure and suture closure included a study which compared primary plastic closure with suture closure after silo formation indicating that the outcome of plastic closure could be affected by the timing of its application [4, 27]. Recently, Bruzoni et al. published the results of randomized controlled trial (RCT) comparing plastic closure with suture closure [28]. Interestingly, these authors reported that plastic closure increased time to full feeding and length of hospital stay. However, the participants were randomized at the timing of hospitalization including and not analyzing separately the outcome according to the timing of closure (primary or staged). To our knowledge, our systematic review is the first comparing plastic closure with suture closure in relation to primary or staged repair of gastroschisis to allow a more comprehensive assessment of the usefulness of plastic closure.
Our analysis indicates that mortality was not influenced by the type of abdominal wall closure (plastic or suture). Mortality rate for each method of closure was 2–4% independently of primary or staged closure. This rate is similar to that reported before in gastroschisis (3–16%) [28].
Wound infection could only be analyzed for the ‘silo’ group. Plastic closure had less incidence of wound infection in silo group. This result is compatible to previous systematic reviews and RCT which included the timing of closure [4, 28]. Suture closure is usually performed in operation room with well sterilized condition, whereas plastic closure is often performed in NICU. Therefore, it is difficult to speculate how this occurs. In addition, there seems to be some variance in the usage of antibiotics, which was not described in detail in every report. As plastic closure is often performed in non-sterilized condition, prolonged antibiotics use may have been administered for plastic closure in some reports. Further investigation and evaluation will be needed for clarification of this issue.
Due to data availability, only initiation of feeding and length of hospital stay were analyzed for primary group. There was no significant difference between groups (Fig. 5). As patients that undergo primary closure are usually relatively stable and have a lower intra-abdominal pressure, both closure methods might have a better clinical course when applied as a primary closure.
Duration of ventilation, time to initiation of feeding, and length of hospital stay were significantly shorter in plastic closure after silo formation. The reduced length of ventilation in plastic closure is in agreement with a previous RCT which included both primary and silo closure [28]. Suture closure is performed in the operating room under general anesthesia, whereas plastic closure is usually performed in NICU without general anesthesia. The absence of general anesthesia may lead to a reduced length of ventilation after plastic closure.
A similar reasoning could apply to the earlier initiation of enteral feeding and reduced length of hospital stay after plastic closure compared to suture repair in children receiving silo insertion. However, although plastic closure had earlier initiation of feeding, there was no significant difference in the time to reach full enteral feeding. The length of hospital stay appears to be dependent of the time needed to reach normal physiological activity, notable by factors such as full enteral feeding. While it is a remarkable finding that plastic closure is associated with a shorter hospital stay, this issue needs further investigation.
There are several limitations in the current evidence. The risk of bias was serious in all outcomes mainly due to bias of confounding factors. More specifically, the method of closure was determined based on surgeons’ preference. Therefore, possible confounding factors such as intra-abdominal pressure could not be excluded. Intra-abdominal pressure was not monitored in most studies despite the possibility that patients with higher intra-abdominal pressure might undergo suture closure, which affects the duration of ventilation. High intra-abdominal pressure caused by edematous and thickened bowel, can potentially prolong the time to reach enteral feeding. Therefore, a serious risk of bias could not be excluded. In the current review, all outcomes in each group did not meet OIS, especially for continuous data. For continuous data, only 18 patients in the ‘primary’ group (5 plastic and 13 suture) and 36 patients in the ‘silo’ group (18 plastic and 18 suture) were included in the analyses, leading to serious imprecision. These limitations in published data resulted in very low quality of evidence. In addition, whereas we performed meta-analyses for primary closure and closure after silo formation separately, there is no definite criteria for the timing of closure. We acknowledge that the timing of closure was affected by the closure method itself. To obtain a higher quality of evidence, a well-designed prospective study is needed.
Conclusion
Current findings suggest that plastic closure may shorten postoperative recovery including the duration of ventilation, time to initiation of enteral feeding, and the length of hospital stay for infants with gastroschisis requiring silo. The evidence of the literature was suboptimal justifying the need for a prospective study.
References
Gamba P, Midrio P (2014) Abdominal wall defects: prenatal diagnosis, newborn management, and long-term outcomes. Semin Pediatr Surg 23:283–290
Owen A, Marven S, Johnson P et al (2010) Gastroschisis: a national cohort study to describe contemporary surgical strategies and outcomes. J Pediatr Surg 45:1808–1816
Sandler A, Lawrence J, Meehan J et al (2004) A “plastic” sutureless abdominal wall closure in gastroschisis. J Pediatr Surg 39:738–741
Youssef F, Gorgy A, Arbash G et al (2016) Flap versus fascial closure for gastroschisis: a systematic review and meta-analysis. J Pediatr Surg 51:718–725
Kunz SN, Tieder JS, Whitlock K et al (2013) Primary fascial closure versus staged closure with silo in patients with gastroschisis: a meta-analysis. J Pediatr Surg 48:845–857
Allin BSR, Tse WHW, Marven S et al (2015) Challenges of improving the evidence base in smaller surgical specialties, as highlighted by a systematic review of gastroschisis management. PLos One 10:e0116908
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of intervention. http://www.handbook.cochrane.org. Accessed 1 Nov 2018
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Miyake H, Seo S, O’Connell J et al (2018) Safety and utility of plastic/sutureless closure for the infants with gastroschisis in different timing of repair: a systematic review and meta-analysis. International prospective register of systematic reviews, PROSPERO. CRD42018086513
Guyatt GH, Oxman AD, Schunemann HJ et al (2011) GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64:380–382
Balshem H, Helfand M, Schunemann HJ et al (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Guyatt GH, Oxman AD, Vist G et al (2011) GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias). J Clin Epidemiol 64:407–415
Guyatt GH, Oxman AD, Montori V et al (2011) GRADE guidelines: 5. Rating the quality of evidence-publication bias. J Clin Epidemiol 64:1277–1282
Guyatt GH, Oxman AD, Kunz RE et al (2011) GRADE guidelines: 6. Rating the quality of evidence-imprecsion. J Clin Epidemiol 64:1283–1293
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol 64:1294–1302
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 8. Rating the quality of evidence-indirectness. J Clin Epidemiol 64:1303–1310
Guyatt GH, Oxman AD, Sultan S et al (2011) GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 64:1311–1316
Sterne JAC, Hernan MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Dupont WD, Plummer WD (1990) Power and sample size calculations: a review and computer program. Control Clin Trial 11:116–128
The GRADE working group. https://gradepro.org/ Accessed 30 Aug 2018
Kandasamy Y, Whitehall J, Gill A et al (2010) Surgical management of gastroschisis in North Queensland from 1988 to 2007. J Pediatr Child Health 46:40–44
Orion KC, Krein M, Liao J et al (2011) Outcomes of plastic closure in gastroschisis. Surgery 150:177–185
McNamara WF, Hartin CW, Escobar MA et al (2011) Outcome differences between gastroschisis repair methods. J Surg Res 165:19–24
Machida M, Takamizawa S, Yoshizawa K et al (2013) Effectiveness of sutureless abdominal wall closure for gastroschisis. Shinshu Igaku 61:27–31
Schlueter RK, Azarow KS, Hines AG et al (2015) Identifying strategies to decrease infectious complications of gastroschisis repair. J Pediatr Surg 50:98–101
Dariel A, Poocharoen W, de Silva N et al (2015) Secondary plastic closure of gastroschisis is associated with a lower incidence of mechanical ventilation. Eur J Pediatr Surg 25:34–40
Choi WW, McBride CA, Bourke C et al (2012) Long-term review of sutureless ward reduction in neonates with gastroschisis in the neonatal unit. J Pediatr Surg 47:1516–1520
Bruzoni M, Jaramillo JD, Dunlap JL et al (2017) Sutureless vs sutured gastroschisis closure: a prospective randomized controlled trial. J Am Colloid Surg 224:1091–1096
Acknowledgements
Dr. Agostino Pierro was supported by the endowment of the Robert M. Filler Chair of Surgery, The Hospital for Sick Children, and by the Canadian Institutes of Health Research (CIHR) Foundation Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Miyake, H., Seo, S., O’Connell, J.S. et al. Safety and usefulness of plastic closure in infants with gastroschisis: a systematic review and meta-analysis. Pediatr Surg Int 35, 107–116 (2019). https://doi.org/10.1007/s00383-018-4381-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4381-7