Abstract
Introduction
Pyloromyotomy is the standard care for hypertrophic pyloric stenosis. The traditional approach for this procedure is a right upper quadrant transverse incision, although other “open” approaches, such as circumumbilical or periumbilical incision have been described. The more recent approach used is laparoscopic pyloromyotomy (LP), but experience feedback is still debated and its benefits remain unproven. The aim of this study was to make a review of all our LP procedures with an objective evaluation according to the literature.
Methods
A retrospective analysis of all the LPs performed in one University Children’s Hospital between 1 January 1996, and 30 December 2015 was realized. Information regarding the patient’s status, intraoperative and postoperative data was analyzed.
Results
407 patients were included in this study. The mean operative time of the overall procedure was 24 ± 13 min, which significantly increased with the length of the pyloric muscle (p = 0.004) and significantly impacted the full feeding time (p = 0.006). 3.4% required conversion to an open procedure during the LP. We observed a significant correlation between conversion for mucosal perforation and weight loss (p = 0.04) and between conversion for mucosal perforation and preoperative weight (p = 0.002). A redo procedure was indicated in 3.7%, for incomplete pyloromyotomy each time. The mean postoperative hospital length of stay for all procedures was 1.6 ± 0.8 days. There were no inflammatory scars. None had incisional hernias or wound dehiscence.
Discussion
LP procedure appeared to be as quick as the open procedure. Our results were similar to others series for intraoperative complications. According to operative time, this technique does not have an impact on operative room utilization. Vomiting duration at presentation in HPS does not seem to have a significant impact on postoperative outcomes. LP procedure causes little pain during the postoperative period. No wound complications were registered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic pyloric stenosis (HPS) affects 2 out of 1000 live births and is the most common surgical cause of nonbilious vomiting in early infancy [1, 2]. Pyloromyotomy is the standard care for this condition. The traditional approach for this procedure is a right upper quadrant transverse incision, although other “open” approaches, such as circumumbilical [3] or periumbilical incision [4], have been described. These approaches provide ease of access and excellent cosmetic results. Although open pyloromyotomy is associated with a low complication risk, it results in scarring that has been associated with reports of a worse body image and less scar cosmetic satisfaction [5].
Since the first description of the laparoscopic pyloromyotomy (LP) approach 25 years ago [6], this technique has been taught extensively throughout the world. Laparoscopic pyloromyotomy has several potential advantages, including a shorter hospital stay, shorter postoperative recovery, less postoperative pain and lower complication rates. This technique is also quick to learn [7]. However, these benefits, as reported in published studies, have been inconsistent [8,9,10,11,12,13]. Disadvantages, including higher risks of incomplete pyloromyotomy and perforation requiring reoperation, have been reported by some studies [14, 15], but have not been confirmed in randomized controlled trials [11, 13]. Currently, LP for HPS management is supported by high level of evidence [16, 17].
The aim of this study was to create a review of all the LP procedures with an objective evaluation according to the literature.
Materials and methods
A retrospective analysis of all the LPs performed in our University Children’s Hospital between 1 January 1996, and 30 December 2015. We made the choice in our center to manage hypertrophic pyloric stenoses only by laparoscopy since 1996. This unit’s decision was supported to improve self-learning and for the resident formation.
The study was approved by the Regional Medical and Ethical Center Institutional Review Board. Patients that underwent pyloromyotomy in addition to other surgical procedures were excluded from the study.
Information regarding the patient’s status (gender, age, weight loss at hospital arrival, weight at the time of surgery and sonographic pyloric length), intraoperative data (incision to dressing, complications) and postoperative data (postoperative hospital length of stay, persistent vomiting, analgesic consumption, redo procedure and time to ad lib feedings) were analyzed.
Complications were classified as being either specific or non-specific in nature. Specific complications included incomplete pyloromyotomy and mucosal perforation. Non-specific complications included fascia dehiscence and wound infections. We also performed a review of our complications according to the Clavien-Dindo classification [18].
General anesthesia protocol
Preoperative management, crush intravenous (IV) induction, and maintenance of anesthesia were standardized in both groups. Correction of preoperative blood electrolytes and metabolic disturbances with appropriate IV fluids was achieved before pyloromyotomy. Surgery was undertaken only if bicarbonate levels were less than 30 mmol/L. After preoxygenation with 100% oxygen and atropine (15 µg/kg IV), a rapid-sequence induction of anesthesia was induced with propofol (5 mg/kg) and succinylcholine (2 mg/kg). After orotracheal intubation, ventilation was mechanically controlled with inhalation of isoflurane in 50% N2O/O2, and atracurium (0.3 mg/kg) was administered to maintain muscle relaxation. Alfentanil (10 µg/kg) was given before the incision. No prophylactic antibiotics were administered at induction.
Surgical technique
Dehydratation and metabolic alkalosis were corrected preoperatively according to protocol in all cases.
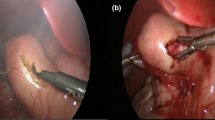
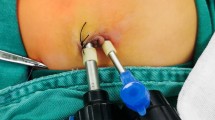
During this period, all procedures were performed in the same manner. A 2 mm 0-degree laparoscope was introduced through a 3-mm trocar placed via a Veres® needle at the right umbilical ring. Pneumoperitoneum was established to a pressure of 4 mmHg; flow 2 mmHg. A laparoscopic atraumatic grasper was inserted directly through the abdominal wall through a short incision without trocar on the right flank. It brought the pylorus and exposed the avascular incision area. An additional 3-mm trocar was placed above the pylorus on the median line. The endoscopic pylorotome was then inserted through it. After pyloric incision, the knife was replaced by a pyloric spreader to complete the pyloromyotomy. Adequacy of the pyloromyotomy was confirmed by insufflating 50 cc of air through a nasogastric tube; the absence of mucosal perforation was thus assessed.
Postoperative care
The postoperative feeding regimen was the same for all patients according to our institutional protocol for postoperative HPS feedings. All patients started the feeding regimen using 30 mL of water 3 h after the procedure. If this was well-tolerated, the infant could then receive 60 mL of formula 3 h later and ad libitum feeding 3 h later. The IV fluid was discontinued, when the infant tolerated two consecutive ad libitum feedings. When vomiting occurred, the next feeding was omitted and was resumed 6 h later at the same dose. All episodes of vomiting were recorded.
The infant was discharged as soon as he was able to tolerate full feeding. Systematic follow-up consisted of a medical examination 1 month after surgery.
Analgesia protocol
Postoperative pain was monitored with the EDIN scale [19], calculated every 3 h during the first 12 h, and then at 18, 24, 36 and 48 h postoperatively. Pain score ranged from 0 (no pain) to 15 (maximum pain). When the pain score was evaluated over 5, the infant received paracetamol (7.5 mg/kg IV). If this administration was not sufficient, nalbuphine hydrochloride (0.2 mg/kg IV) was then used.
Statistical analysis
The former collected data were described by mean and standard deviation for quantitative variables, and numbers and percentages for qualitative variables. Statistical analyses were performed using Epi Info™ 7.2 software. Statistical comparisons were made using the Wilcoxon and Mann–Whitney’s tests with a p value < 0.05 considered statistically significant.
Results
During the study period, 410 patients underwent LP and 407 were included in this study. 3 patients were excluded because of the lack of data. Nine surgeons were reported during this period as main operators. We recorded a regurgitation history before HPS history in 25% of cases. The duration of pre-hospitalization vomiting was 7 ± 6 days and the preoperative weight loss was heterogeneous (97 ± 127 g).
We recorded seasonality with a higher rate of HPS in spring and autumn (35 and 40%, respectively) than in winter (10%) and in summer (15%) (p = 0.64). The average birth term was 39 ± 2 weeks of amenorrhea. We found a familial pyloric stenosis history in 3.7% and an associated history in 13%. All epidemiological data were illustrated in Table 1.
The mean operative time of the overall procedure was 24 ± 13 min. This time was significantly increased with the length of the pyloric muscle (p = 0.004) and significantly impacted the full feeding time (p = 0.006).
14 cases (3.4%) required conversion to an open procedure during the LP. In 2/3 of cases, a mucosal perforation during the laparoscopic procedure was involved (2.3%); exposure difficulties explained the conversion in 1/3 of cases. There was no significant complication rate between each surgeon. We observed a significant correlation between conversion for mucosal perforation and weight loss (p = 0.04) and between conversion for mucosal perforation and preoperative weight (p = 0.002).The lower the weight or the greater the weight loss, the more the mucosal perforation rate increased. A redo procedure was indicated in 3.7% (15 cases) for incomplete pyloromyotomy each time. A laparoscopic approach was used in all cases and was successful. All complications, according to Clavien-Dindo classification were reported in Table 2.
Concerning postoperative management, the use of grade II analgesics (nalbuphine hydrochloride) was necessary in 0.7% of cases (n = 3). No grade III were used in any of the cases. Paracetamol (grade I) was given after the procedure according to pain evaluation. The rate of paracetamol administration was 1.5 ± 1.4 during hospital stay.
The full feeding delay was evaluated at 38 ± 11 h without significant correlation to other data, particularly operative time (p = 0.51).
The postoperative emesis rate was evaluated at 29.9%. The operative time had no incidence on postoperative emesis, even though we recorded a tendency to decrease in emesis according to operative time (p = 0.09). Thirteen patients were re-admitted to the hospital for emesis. The radiological findings excluded an insufficient pyloromyotomy, and medical treatment was successfully administered in all cases.
The mean postoperative hospital length of stay for all procedures was 1.6 ± 0.8 days. According to the follow-up, all patients had normal digestion at 1 month. There were no inflammatory scars. None had incisional hernias or wound dehiscence. All patients had gained weight since they had been discharged. 24% (n = 98) had some gastroesophageal reflux 1 month after the LP procedure.
Discussion
The introduction of laparoscopic pyloromyotomy by Alain et al. [6] in 1991 has led to many discussions about its additional value. The laparoscopic utilization for pyloric stenosis management is currently a matter of debate and the benefits remain unproven.
Our results in this study were similar to others: the mean operative time was less than 25 min as for Van der bilt et al. [20] and the LP procedure appeared to be as quick as the open procedure. This observation is the same in several meta-analyses [11, 21, 22]. According to the operative time, this technique does not have an impact on operative room utilization. We showed a significant correlation between operative time and the length of the pyloric muscle. Ostlie et al. [23] suggested that an LP incision length of approximately 2 cm would ensure a complete pyloromyotomy. They also suggested that at the very least the pyloromyotomy should be longer than the length of the pyloric channel measured by ultrasound [24]. Beyond considering the pyloric muscle’s length, exposition difficulties related to the pyloric muscle’s volume and the struggles of grasping, it could also explain the significant increase of the operative time.
Concerning perioperative complications, LP was criticized for two major points: incomplete pyloromyotomy and mucosal perforation.
While it is rare after an open procedure, incomplete pyloromyotomy after LP has an incidence ranging from 1.4 to 5.6% according to literature [20, 25,26,27,28,29]. Our results are similar with an incomplete pyloromyotomy rate at 3.7%.
This complication has been previously reported to be determined by case volume and surgical experience [20, 25, 30], nonetheless, we brought to light in a previous study in our center that this complication did not depend on the surgeon or his experience [7]. Sathya et al. described recently that there is no significant difference in complication rates when comparing LP to the open procedure, even though LP seems to be associated with a higher rate of inadequate pyloromyotomy [22]. The absence of direct digital palpation increases the risks of both incomplete pyloromyotomy and mucosal perforation.
Mucosal perforation associated with the LP approach creates a debate on its utilization. Literature has shown that LP is associated with an increased rate of mucosal perforation, presumed by conflicting retrospective and prospective data [11]. However, this observation was controversial, with some series that showed a higher incidence of perforation with LP [15, 25], and others who reported no difference or even a lower rate with LP [14, 29, 31]. The Sola and Neville’s meta-analysis demonstrated no statistically significant difference in mucosal perforation rates between the two operative techniques [11]. We reported a mucosal perforation rate of 2.3%. Mucosal perforation after LP has an incidence ranging from 1.3 to 3.3% according to recent literature [11, 13, 21]. Our result for this complication is in agreement with the observations of others. A recent systematic review and meta-analysis comparing laparoscopic versus open pyloromyotomy concluded that there was no significant difference between these two groups in regard to postoperative complications, which included intraoperative perforation and the need for reoperation [22].
We noticed that preoperative weight had a significant impact on mucosal perforation and weight loss. This observation brought to light the impact of low weight, which could be correlated with a lower amount of workspace for laparoscopy. Exposure difficulties related to this inferior amount of workspace could explain the reason of mucosal perforation rather than the general health status of the child. All mucosal perforations in our series were intraoperative emphasis and were managed by an umbilical open procedure, which succeeded without any complication. Naturally, this argument must be balanced by the fact that the weight loss is calculated between the weight at admission and the last known weight recorded previously in the external follow-up.
Regarding postoperative management, different criterias should be analyzed.
First of all, concerning postoperative vomiting: Al-Jazaeri et al. [32] show that the vomiting duration at presentation in HPS does not seem to have a significant impact on postoperative outcomes. Likewise, we don’t show any correlation between the preoperative vomiting duration and postoperative outcome. However, we find a significant correlation between preoperative weight loss and ad libitum feeding time, which is directly correlated with postoperative emesis (p = 0.03). The operative time is also significantly correlated with postoperative emesis. Lee et al. [33] find that in pyloromyotomy, there is an increased risk of prolonged hospitalization for low weight patients as well as for those who receive lower preoperative hydration. A longer anesthesia period according to the operative time could explain this observation, as well as the length of pneumoperitoneum time. Postoperative nausea and vomiting is a common and well-known complication of laparoscopic procedures [34].
Second, the ad libitum feeding time: The full feeding delay in our series (38 h) is compatible with the literature, without significant connection to other data. The feeding protocol we used could be criticized. Indeed, even if the children do not vomit, the minimum full feeding time takes 35 h. Other feeding protocols revealed lower time to ad libitum feeding, with rates ranging from 19 to 35 h [12, 14, 15, 35, 36]. A double-blinded multicenter randomized controlled trial studying recovery after open versus laparoscopic pyloromyotomy [15] brings to light a significantly better recovery after the LP procedure, but no difference concerning postoperative vomiting. Furthermore, other studies show no advantage of using a feeding protocol in comparison to ad libitum feeds for infants who have undergone laparoscopic pyloromyotomy [37]. Perhaps we could have adapted the postoperative feeding protocol according to weight loss with a longer postoperative fasting period even if as Adibe et al. described [37], a standardized feeding regimen offers no advantage over ad libitum feeds for infants who have undergone laparoscopic pyloromyotomy. Other studies bring to light that feeding within 4 h postoperatively leads to more severe vomiting than if the feeding begins later. Vomiting leads to the child’s discomfort, parental anxiety, prolonged time required to achieve full oral feeding and a prolonged postoperative hospital stay [38]. Luciani et al. proposed systematic medical treatment to prevent postoperative vomiting in high-risk children, to decrease hospital stay [39]. According to our experience and literature, systematic medical treatment and the delay before starting postoperative feeding could be applied for patients with a high risk of postoperative emesis. Leclair et al. show in their feeding protocol [14], the use of prokinetics (domperidone 1 mg every 8 h) after 2 two consecutive vomiting episodes.
Third, we note that this LP procedure causes little/less pain during the postoperative period. Nalbuphine hydrochloride was erratically prescribed and paracetamol administration was registered less than twice. This technique ensured comfort for several children. Reduction of postoperative pain has been described in literature, with a shorter hospital length of stay and an earlier reestablishment of normal activity [1]. Compared to the open procedure, infant management by the LP procedure needs a lower number of doses of analgesic drugs [15]. In our experience, children after LP were calmer after food intake, than after drug administration.
In our series, no wound complications were registered. There were no infectious complications, dehiscence scars, or hernia. In all the studies which reported wound complications, the rate was lower for the LP approach [11]. The major advantage of laparoscopic approach is in wound cosmetics, according to the small sized incisions. Wound infection risks have been reported in association with the single periumbilical incision for the open pyloromyotomy. However, no study has been published on this subject. Laparoscopy is well-known for its lack of wound complications and the improvement concerning cosmetic appearance [40]. Rumsey et al. indicated that the cosmetic appearance following LP is better than the one of the open procedure in which the scar remains quite visible and may be a cause of distress for patients later in life [41]. But, this observation seems to have some limits according to the fact that there is no prospective study, which compares LP, to open technics regarding cosmetic results.
Concerning gastroesophageal reflux, before surgery (25%) and after surgery (24%), this rate is similar to the overall population of the same age (28.7%) [42]. This observation could hold harmless gastroesophageal reflux in the etiology of pyloric stenosis. Some causes were involved in pyloric stenosis without certainty. Our results confirm the higher risk of pyloric stenosis in boys than in girls, which is the first recorded and most consistent epidemiologic feature of the disease. We found the same results as in the literature (5 males for 1 female) [43]. Recurrence risk in families and genetic etiology were mentioned. Many casereports of pyloric stenosis have been stimulated by the occurrence of more than one affected member in the family [43, 44].
Conclusion
In summary, this is the largest retrospective single center pilot study in which laparoscopic pyloromyotomy for pyloric stenosis appears to be performed safely with equivalent rates of mucosal perforation according to other procedures. LP is painless, with complete recovery time depending on the postoperative feeding protocol.
The technique’s choice (laparoscopic or open procedure) is a matter of habit and depends on the surgery and anesthetic unit’s custom. LP currently appears to be as safe and efficient as the open procedure and the wound debate remains unstudied.
References
Cascio S et al (2013) Hypertrophic pyloric stenosis in premature infants: evaluation of sonographic criteria and short-term outcomes. Pediatr Surg Int 29(7):697–702
Stark CM et al (2015) Association of prematurity with the development of infantile hypertrophic pyloric stenosis. Pediatr Res 78(2):218–222
Tan KC, Bianchi A (1986) Circumumbilical incision for pyloromyotomy. Br J Surg 73(5):399
Lazar D et al (2008) Transumbilical pyloromyotomy with umbilicoplasty provides ease of access and excellent cosmetic results. J Pediatr Surg 43(7):1408–1410
Siddiqui S et al (2012) Pyloromyotomy: randomized control trial of laparoscopic vs open technique. J Pediatr Surg 47(1):93–98
Alain JL, Grousseau D, Terrier G (1991) Extramucosal pyloromyotomy by laparoscopy. Surg Endosc 5(4):174–175
Binet A, Bastard F, Meignan P, Braïk K, Touze AL, Villemagne T, Morel B, Robert M, Klipfel C, Lardy H (2017) Laparoscopic pyloromyotomy: a study of the learning curve. Eur J Pediatr Surg. https://doi.org/10.1055/s-0037-1603090
Carrington EV et al (2012) Cost-effectiveness of laparoscopic versus open pyloromyotomy. J Surg Res 178(1):315–320
Hall NJ et al (2004) Meta-analysis of laparoscopic versus open pyloromyotomy. Ann Surg 240(5):774–778
Oomen MW et al (2010) Learning curves for pediatric laparoscopy: how many operations are enough? The Amsterdam experience with laparoscopic pyloromyotomy. Surg Endosc 24(8):1829–1833
Sola JE, Neville HL (2009) Laparoscopic vs open pyloromyotomy: a systematic review and meta-analysis. J Pediatr Surg 44(8):1631–1637
St Peter SD et al (2006) Open versus laparoscopic pyloromyotomy for pyloric stenosis: a prospective, randomized trial. Ann Surg 244(3):363–370
Jia WQ et al (2011) Open versus laparoscopic pyloromyotomy for pyloric stenosis: a meta-analysis of randomized controlled trials. Eur J Pediatr Surg 21(2):77–81
Leclair MD et al (2007) Laparoscopic pyloromyotomy for hypertrophic pyloric stenosis: a prospective, randomized controlled trial. J Pediatr Surg 42(4):692–698
Hall NJ et al (2009) Recovery after open versus laparoscopic pyloromyotomy for pyloric stenosis: a double-blind multicentre randomised controlled trial. Lancet 373(9661):390–398
Zani-Ruttenstock E et al (2015) Are paediatric operations evidence based? A prospective analysis of general surgery practice in a teaching paediatric hospital. Pediatr Surg Int 31(1):53–59
Ballouhey Q et al (2016) Differential learning processes for laparoscopic and open supraumbilical pyloromyotomy. Pediatr Surg Int 32(11):1047–1052
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205
Debillon T et al (2001) Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal Edn 85(1):F36–F41
van der Bilt JD et al (2004) Laparoscopic pyloromyotomy for hypertrophic pyloric stenosis: impact of experience on the results in 182 cases. Surg Endosc 18(6):907–909
Hall NJ et al (2014) Risk of incomplete pyloromyotomy and mucosal perforation in open and laparoscopic pyloromyotomy. J Pediatr Surg 49(7):1083–1086
Sathya C et al (2017) Laparoscopic versus open pyloromyotomy in infants: a systematic review and meta-analysis. Pediatr Surg Int 33(3):325–333
Ostlie DJ et al (2004) An effective pyloromyotomy length in infants undergoing laparoscopic pyloromyotomy. Surgery 136(4):827–832
St Peter SD, Ostlie DJ (2008) Pyloric stenosis: from a retrospective analysis to a prospective clinical trial—the impact on surgical outcomes. Curr Opin Pediatr 20(3):311–314
Adibe OO et al (2006) Comparison of outcomes after laparoscopic and open pyloromyotomy at a high-volume pediatric teaching hospital. J Pediatr Surg 41(10):1676–1678
Campbell BT et al (2002) A comparison of laparoscopic and open pyloromyotomy at a teaching hospital. J Pediatr Surg 37(7):1068–1071 (discussion 1068–1071)
Hall NJ et al (2004) Retrospective comparison of open versus laparoscopic pyloromyotomy. Br J Surg 91(10):1325–1329
Sitsen E, Bax NM, van der Zee DC (1998) Is laparoscopic pyloromyotomy superior to open surgery? Surg Endosc 12(6):813–815
Yagmurlu A et al (2004) Comparison of the incidence of complications in open and laparoscopic pyloromyotomy: a concurrent single institution series. J Pediatr Surg 39(3):292–296 (discussion 292–296)
Ford WD, Crameri JA, Holland AJ (1997) The learning curve for laparoscopic pyloromyotomy. J Pediatr Surg 32(4):552–554
Kim SS et al (2005) Pyloromyotomy: a comparison of laparoscopic, circumumbilical, and right upper quadrant operative techniques. J Am Coll Surg 201(1):66–70
Al-Jazaeri A et al (2011) Can the duration of vomiting predict postoperative outcomes in hypertrophic pyloric stenosis? Ann Saudi Med 31(6):609
Lee SL, Stark R (2011) Can patient factors predict early discharge after pyloromyotomy? Perm J 15(2):44
Cohen MM et al (1994) The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg 78(1):7–16
GREASON KL et al (1997) A prospective, randomized evaluation of laparoscopic versus open pyloromyotomy in the treatment of infantile hypertrophic pyloric stenosis. Pediatr Endosurg Innov Tech 1(3):175–179
Fujimoto T et al (1999) Laparoscopic extramucosal pyloromyotomy versus open pyloromyotomy for infantile hypertrophic pyloric stenosis: which is better? J Pediatr Surg 34(2):370–372
Adibe OO et al (2007) Ad libitum feeds after laparoscopic pyloromyotomy: a retrospective comparison with a standardized feeding regimen in 227 infants. J Laparoendosc Adv Surg Tech A 17(2):235–237
van der Bilt JD et al (2004) Early feeding after laparoscopic pyloromyotomy: the pros and cons. Surg Endosc 18(5):746–748
Luciani JL et al (1997) Prognostic factors of the postoperative vomiting in case of hypertrophic pyloric stenosis. Eur J Pediatr Surg 7(2):93–96
Attwood SE et al (1992) A prospective randomized trial of laparoscopic versus open appendectomy. Surgery 112(3):497–501
Rumsey N, Harcourt D (2004) Body image and disfigurement: issues and interventions. Body Image 1(1):83–97
Martigne L et al (2009) P. 43 Prévalence du reflux gastro-œsophagien (RGO) chez l’enfant et l’adolescent en France: résultats d’une étude observationnelle transversale. Gastroentérol Clin Biol 33(3):A40
MacMahon B (2006) The continuing enigma of pyloric stenosis of infancy: a review. Epidemiology 17(2):195–201
Mc KT, Mac MB, Record RG (1951) The familial incidence of congenital pyloric stenosis. Ann Eugen 16(3):260–281
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Binet, A., Klipfel, C., Meignan, P. et al. Laparoscopic pyloromyotomy for hypertrophic pyloric stenosis: a survey of 407 children. Pediatr Surg Int 34, 421–426 (2018). https://doi.org/10.1007/s00383-018-4235-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-018-4235-3