Abstract
Aims and objectives
The high morbidity and mortality rates in congenital diaphragmatic hernia (CDH) are attributed primarily to severe lung hypoplasia and/or persistent pulmonary hypertension (PPH). PPH in CDH is characterized by abnormal vascular remodeling with thickening of medial and adventitial layers and extension of smooth muscle into previously nonmuscularized arteries. Excessive proliferation of pulmonary arterial smooth muscle cells (PASMC) is an important contributor to the concentric pulmonary arterial remodeling. An increase in cytosolic-free Ca2+ concentration in PASMC is a major trigger for pulmonary vasoconstriction and a key stimulus for PASMC proliferation and migration. Calcium-sensing receptor (CaSR), a member of the G-protein coupled receptor family, is activated by cations (e.g., Ca2+, Mg2+) and polyamines. Under normal physiological conditions, the expression levels of CaSR in the pulmonary vasculature are very low. Canonical transient receptor potential channels (TRPCs) constitute a series of nonselective cation channels with variable degree of Ca2+ selectivity. TRPC6 has been reported to play a crucial role in the regulation of neo-muscularization, vasoreactivity, and vasomotor tone in the pulmonary vasculature. We hypothesized that CaSR and TRPC6 expression is upregulated in the pulmonary vasculature of nitrofen-induced CDH rats.
Materials and methods
Following ethical approval (REC1103), time-pregnant Sprague Dawley rats received nitrofen or vehicle on gestational day (D) 9. D21 fetuses were divided into CDH and control (n = 12). Quantitative real-time polymerase chain reaction (QRT-PCR), western blotting, and confocal-immunofluorescence microscopy were performed to detect lung gene and protein expression of CaSR and TRPC6.
Results
QRT-PCR and western blot analysis revealed that CaSR and TPRC6 expression was significantly increased in the CDH group compared to controls (p < 0.05). Confocal-immunofluorescence microscopy revealed that CaSR and TRPC6 lung expression was markedly increased in CDH group compared to controls.
Conclusion
Increased CaSR and TRPC6 expression in CDH lung suggests that CaSR interacting with TRPC6 may contribute to abnormal vascular remodeling resulting in pulmonary vasoconstriction and development of PPH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite remarkable progress in resuscitation and intensive care, the morbidity and mortality rates in congenital diaphragmatic hernia (CDH) remain high [1]. Most newborns with CDH die primarily of severe lung hypoplasia and/or pulmonary hypertension [1]. Pulmonary hypertension in CDH is characterized by excessive pulmonary vascular remodeling with hypertrophy of medial and adventitial layers and extension of smooth muscle into previously nonmuscularized arteries [2]. Excessive proliferation of pulmonary arterial smooth muscle cells (PASMC) is an important contributor to the concentric pulmonary arterial remodeling [2]. Abnormal pulmonary vascular remodeling occurs as a result of increased PASMC proliferation and decreased PASMC apoptosis [3].
Many intracellular signaling cascades are involved in the regulation of PASMC proliferation and migration [3]. An increase in cytosolic-free Ca2+ concentration in PASMC is a major trigger for pulmonary vasoconstriction and a key stimulus for PASMC proliferation and migration [4]. PASMC from patients with idiopathic pulmonary hypertension and experimental animals with pulmonary hypertension have increased resting Ca2+ and enhanced Ca2+ influx [4]. Enhanced Ca2+ influx into PASMC due to upregulation of membrane receptors and/or Ca2+ channels has been shown to result in PASMC contraction and proliferation and to pulmonary vasocontraction and pulmonary vascular modeling [4]. Calcium-sensing receptor (CaSR), a member of the G-protein coupled receptor family, can be activated by cations (e.g., Ca2+, Mg2+) and polyamines [2, 4]. Under normal physiological conditions, the expression levels of CaSR in the pulmonary vasculation are very low [4]. Recently, it was reported that CaSR expression is upregulated and extracellular Ca2+ induced increase in [Ca2+]cyt is enhanced in PASMC from patients with idiopathic pulmonary hypertension [4].
Canonical transient receptor potential channels (TRPCs) are expressed in almost every human tissue including pulmonary vasculative [3, 5]. TRPCs constitute a series of nonselective cations channels with variable degree of Ca2+ selectivity [4]. TRPC6 has been reported to play a crucial role in the regulation of neo-muscularization, vasoreactivity, and vasomotor tone in the pulmonary vasculature [5]. Recently, Tang et al. [6] reported that CaSR functionally interacts with TRPC6 channels to mediate extracellular Ca2+ induced Ca2+ influx and increase the [Ca2+]cyt in PASMC from patients with pulmonary hypertension and animals with experimental pulmonary hypertension.
Nitrofen is an herbicide that has been widely used for many years to create a teratogenic model of CDH [7]. Nitrofen administration to pregnant rodents between day 8 and 11 after conception results in a high rate of CDH, associate pulmonary hypoplasia, and pulmonary vascular abnormalities to their embryos, all remarkably similar to the human malformation [7].
We designed this study to investigate the hypothesis that the pulmonary vascular expression of CaSR and TRPC6 is upregulated in the nitrofen-induced congenital diaphragmatic hernia.
Materials and methods
Following ethical approval (REC1103), time-pregnant Sprague Dawley rats (Harlan Laboratories, Shadlow, UK) were randomly divided into two experimental groups (“CDH” and “control”). The observation of spermatozoids in the vaginal smear was considered as proof of pregnancy and the day of observation was determined as gestational day 0 (D0). On D9, the CDH group received 100 mg of nitrofen (2,4-dichloro-p-nitrophenyl ether, WAKO Chemicals, Osaka, Japan) intragastrically dissolved in 1 ml of olive oil. Animals in the control group received only vehicle. On D21, dams were anesthetized with 2% volatile isoflurane (Piramal Healthcare UK, Morpeth, United Kingdom). An intracardiac bolus of Pentobarbital Sodium into the dam’s heart was performed to sacrifice the dam and her fetuses at the same time. Subsequently, fetuses were delivered via cesarean section. The fetuses were divided according to the two experimental groups. After thoracotomy, D21 fetuses were inspected for CDH. Lungs with a diaphragmatic defect and controls were dissected and stored native either at − 80 °C, in formalin, or in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) at − 20 °C. All animal experiments were performed according to the current guidelines for management and welfare of laboratory animals. The Health Products Regulatory Authority approved the protocol of these animal experiments (ref. AE19127/P026) under the Cruelty to Animals Act 1876 (as amended by European Union [Protection of Animals Used for Scientific Purposes] Regulations 2012).
RNA isolation from lung (D21)
TRIzol reagent (Invitrogen) was used for the acid guanidinium thiocyanate–phenol–chloroform extraction method to isolate total RNA from D21 lung (n = 12) according to the manufacturer’s protocol. Spectrophotometrical quantification of total RNA was performed using a NanoDrop ND-1000 UV–Vis spectrophotometer (Thermo Scientific Fisher, Wilmington, USA). The RNA solution was stored at − 20 °C until further use.
cDNA synthesis and quantitative polymerase chain reaction
Reverse transcription of total RNA was carried out at 85 °C for 3 min (denaturation), at 44 °C for 60 min (annealing) and at 92 °C for 10 min (reverse transcriptase inactivation) using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche Diagnostics, West Sussex, UK) according to the manufacturer’s instruction. The resulting cDNA was used for quantitative real-time polymerase chain reaction (qRT-PCR) using a LightCycler 480 SYBR Green I Master (Roche Diagnostics, Mannheim, Germany) in a total reaction mix of 20 µl per well. After 5 min of initial denaturation at 95 °C, 55 cycles of amplification for each primer were carried out. Each cycle included denaturation at 95 °C for 10 s, annealing at 60 °C for 15 s, and elongation at 72 °C for 10 s. Relative mRNA levels of gene expression were determined using a LightCycler 480 System (Roche Diagnostics) and the relative changes in gene expression level of CaSR and TRPC6 were normalized against the level of GAPDH gene expression in each sample (DDCT method). Experiments were carried out in duplicate for each sample and primer.
Western blot
Fresh frozen lungs (n = 6 for each group) were thawed, sonicated and proteins were isolated in lysis buffer containing 25 mM Tris–HCl, 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1% NP-40, 10% glycerol, and 1% protease inhibitor cocktail (Sigma–Aldrich, Ireland) prior to gel loading. Gel electrophoresis for protein separation was performed using precast 10% SDS polyacrylamide gels (NuPAGE NovexBis-Tris gels, Invitrogen) in NuPAGE MES SDS running buffer (Invitrogen). Proteins were then transferred to 0.45-lm nitrocellulose membranes (Millipore Corporation, Billerica, USA) by western blotting. Following western blotting, the membranes were blocked in 3% BSA + 0.05% Tween for 30 min or overnight before antibody detection. Primary antibodies against CaSR (rabbit polyclonal, ab18200, 1:500 dilution in PBST, abcam, United Kingdom) and TRPC6 (rabbit polyclonal, ab12249, 1:500 dilution in PBST, abcam, United Kingdom) were incubated overnight at 4 °C. On the next day, followed by extensive washing (4 h), the membranes were incubated with the secondary antibodies in a dilution of 1:5000 followed again by extensive washing. Detection was performed with a PIERCE chemiluminescence kit (Thermo, Fisher Scientific, Dublin, Ireland). GAPDH (Anti-GAPDH antibody, ab9484, Abcam UK) were used to control and equal loading and transfer of the samples.
Immunofluorescence staining and confocal microscopy
Fetal lungs (n = 4 for each group) were fixed with 10% buffered formalin (Santa Cruz Biotechnology) overnight. Whole organs were washed overnight in PBS, embedded in OCT Mounting Compound (VWR International, Leuven, Belgium) and frozen at – 80 °C. Frozen blocks were sectioned transversely at a thickness of 10 µm and mounted on Super Frost Plus slides (VWR international). After washing with PBS sections underwent cell membrane permeabilization with 1% Triton X-100 for 20 min at room temperature. All sections were then washed in PBS + 0.05% Tween (Sigma Aldrich, Saint Louis, USA) and blocked with 3% bovine serum albumin (Sigma Aldrich, Saint Louis, USA) for 30 min to avoid non-specific absorption of immunoglobulin. Sections were incubated with primary antibodies against CaSR (rabbit polyclonal, ab18200, 1:100 dilution in PBST, abcam, United Kingdom), TRPC6 (rabbit polyclonal, ab12249, 1:100 dilution in PBST, abcam, United Kingdom), and alpha smooth muscle actin (α-SMA, mouse monoclonal, M0851, dilution 1:400 in PBST, DAKO Diagnostics Ireland) overnight at 4 °C. After washing, the sections were incubated with corresponding secondary antibodies for 1 h at room temperature. After washing, sections were counterstained with DAPI antibody (1:1000 in PBST, Roche), washed again, and mounted using Sigma Mounting Medium (Sigma–Aldrich, St. Louis, MO, USA). Sections were scanned with a ZEISS LSM 700 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany) and evaluated independently by two investigators.
Statistical analysis
All numerical data are presented as mean ± standard error of the mean. Student’s t test was used for evaluation of differences between two normal distributed groups on D21. The confidence interval was set at 95%.
Results
Relative mRNA expression levels of CaSR and TPRC6
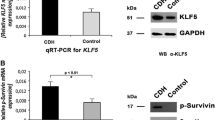
The relative mRNA expression levels of CaSR and TPRC6 were significantly increased (p < 0.05) in lung of CDH group compared to lung of control animals (Fig. 1).
Western blot for CaSR and TPRC6
We analyzed the protein expressions of CaSR and TPRC6 to confirm the qRT-PCR results. Our western blot results showed increased lung protein expression of CaSR and TPRC6 in the CDH group compared to controls (Fig. 2). Equal loading of electrophoresis gels was confirmed by GAPDH staining of the stripped membranes.
Immunofluorescence evaluation of CaSR and TPRC6
Confirmation by western blotting of altered CaSR and TPRC6 expressions was followed by immunofluorescence evaluation of lung tissue. Confocal microscopy showed increased CaSR and TPRC6 expression in CDH lung compared to normal lung tissue (Fig. 3).
Discussion
Multiple mechanisms underlie the development of PPH in CDH patients. Characteristic features of PPH include endothelial dysfunction, enhanced pulmonary artery smooth muscle cell (PASMC) proliferation, and suppressed apoptosis, resulting in an increased pulmonary resistance [8]. The results of the present study show that the expression of both CaSR and TRPC6 is increased in the lung of CDH animal model. It has previously been reported that both CaSR and TRPC6 are upregulated in PASMC in patients with idiopathic pulmonary hypertension and in animal models with pulmonary hypertension [4, 6].
Ca2+ entry from the extracellular space, acting as a crucial mediator, is implicated in vasoconstriction [through its pivotal effect on pulmonary artery smooth muscle cells (PASMCs) contraction] and vascular remodeling (through its stimulatory affect of PASMC proliferation) [9]. Application of extracellular Ca2+ increased [Ca2+]cyt in PASMC isolated from idiopathic pulmonary arterial hypertension patients compared to PASMC from control patients and patients with chronic thromboembolic pulmonary hypertension [10]. Overexpression of CaSR augmented the extracellular Ca2+-induced increase in [Ca2+]cyt and enhanced proliferation in normal PASMC [10]. Yamamura et al. [10] reported that increased expression of CaSR and subsequently enhanced CaSR-mediated [Ca2+]cyt increase contribute to the enhanced Ca2+ signaling and proliferation of PASMC in patients with idiopathic pulmonary arterial hypertension.
Overexpression of CaSR or TPRC6 in normal PASMC results in enhanced extracellular Ca2+-induced increase in [Ca2+]cyst; however, overexpression of both CaSR and TRPC6 markedly increases extracellular Ca2+-induced increase in [Ca2+]cyst compared to either alone [6]. These data suggest that CaSR is functionally coupled to TRPC6 in PASMC and that receptor-and store-operated Ca2+ entry via CaSR-mediated activation of TRPC6 is an important signaling cascade which leads to PASMC contraction, proliferation, and migration in patients with PPH [4]. Controlling the balance between cell proliferation and apoptosis in pulmonary vascular adventia, media and endothelium are essential for maintaining the normal structural and functional integrity of the pulmonary vasculature [4]. Increased PASMC proliferation and decreased PASMC lead to abnormal vascular remodeling resulting in advential and medial thickening causing vasoconstriction.
The present study showed that the expressions of both CaSR and TRPC6 were increased in the lung of CDH animal model. These results suggest that upregulation of CaSR and TRPC6 expression may be an important pathogenic mechanism involved in the development of abnormal vascular remodeling resulting in pulmonary vasoconstriction and PPH.
In summary, the present study demonstrates an altered gene and protein expression of CaSR and TRPC6 in the pulmonary vasculature of nitrofen-induced CDH. Upregulation of CaSR and TRPC6 may contribute to abnormal vascular remodeling resulting in PH in this model. Future studies investigating specific blockers of CaSR and TRPC6 may provide more effective therapies in the management of PPH in CDH.
References
Keijzer R, Puri P (2010) Congenital diaphragmatic hernia. Semin Pediatr Surg 19:180–185
Kool H, Mous D, Tibboel D, de Klein A, Rottier RJ (2014) Pulmonary vascular development goes awry in congenital lung abnormalities. Birth Defects Res C Embryo Today 102:343–358
Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D et al (2015) New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest 147:529–537
Smith KA, Ayon RJ, Tang H, Makino A, Yuan JX (2016) Calcium-sensing receptor regulates cytosolic [Ca2+] and plays a major role in the development of pulmonary hypertension. Front Physiol 7:517
Weber EW, Han F, Tauseef M, Birnbaumer L, Mehta D, Muller WA (2015) TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J Exp Med 212:1883–1899
Tang H, Yamamura A, Yamamura H, Song S, Fraidenburg DR, Chen J et al (2016) Pathogenic role of calcium-sensing receptors in the development and progression of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 310:L846–L859
Guarino N, Shima H, Puri P (2001) Structural immaturity of the heart in congenital diaphragmatic hernia in rats. J Pediatr Surg 36:770–773
Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O et al (2004) Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101:13861–13866
Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX (2012) New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. Am J Physiol Heart Circ Physiol 302:H1546–H1562
Yamamura A, Guo Q, Yamamura H, Zimnicka AM, Pohl NM, Smith KA et al (2012) Enhanced Ca(2+)-sensing receptor function in idiopathic pulmonary arterial hypertension. Circ Res 111:469–481
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nakamura, H., Zimmer, J., Lim, T. et al. Increased CaSR and TRPC6 pulmonary vascular expression in the nitrofen-induced model of congenital diaphragmatic hernia. Pediatr Surg Int 34, 211–215 (2018). https://doi.org/10.1007/s00383-017-4191-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-017-4191-3