Abstract
Objective
To evaluate outcomes and predictors of survival of pediatric thyroid carcinoma, specifically papillary thyroid carcinoma.
Methods
SEER was searched for surgical pediatric cases (≤20 years old) of papillary thyroid carcinoma diagnosed between 1973 and 2011. Demographics, clinical characteristics, and survival outcomes were analyzed using standard statistical methods. All papillary types, including follicular variant, were included.
Results
A total of 2504 cases were identified. Overall incidence was 0.483/100,000 persons per year with a significant annual percent change (APC) in occurrence of 2.07 % from baseline (P < 0.05). Mean age at diagnosis was 16 years and highest incidence was found in white, female patients ages 15–19. Patients with tumor sizes <1 cm more likely received lobectomies/isthmusectomies versus subtotal/total thyroidectomies [OR = 3.03 (2.12, 4.32); P < 0.001]. Patients with tumors ≥1 cm and lymph node-positive statuses [OR = 99.0 (12.5, 783); P < 0.001] more likely underwent subtotal/total thyroidectomy compared to lobectomy/isthmusectomy. Tumors ≥1 cm were more likely lymph node-positive [OR = 39.4 (16.6, 93.7); p < 0.001]. Mortality did not differ between procedures. Mean survival was 38.6 years and higher in those with regional disease. Disease-specific 30-year survival ranged from 99 to 100 %, regardless of tumor size or procedure. Lymph node sampling did not affect survival.
Conclusions
The incidence of pediatric papillary thyroid cancer is increasing. Females have a higher incidence, but similar survival to males. Tumors ≥1 cm were likely to be lymph node-positive. Although tumors ≥1 cm were more likely to be resected by subtotal/total thyroidectomy, survival was high and did not differ based on procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid carcinoma only represents 0.5–3 % of all pediatric malignancies [1], but it still remains the most common endocrine malignancy in children and adolescents [2]. Specifically, papillary thyroid cancer (PTC) is the most common histologic variant [3–5] and has the highest incidence [4]. During its initial presentation, PTC behaves clinically different in children and adolescents when compared to adults [6, 7]. Although only 8 % of pediatric thyroid cancers as a whole present with distant metastasis [8], PTC in children presents with cervical lymph node involvement and distant lung metastasis at a higher rate [4, 9] at first presentation compared to adults. When initially diagnosed, up to 90 % of cases present with palpable lymph nodes in the neck [10].
Despite the tendency for PTC in children to present at a more advanced stage, studies have shown that pediatric patients have a better prognosis compared to adults [11–13]. In contrast, there is a significantly higher mortality in adults with PTC, especially in those with distant spread [14]. These inconsistent observations of advanced disease stages with rather good prognoses have made it difficult for clinicians to agree upon an optimal surgical treatment approach [5]. Large institutions across the country have had stark differences in their standard treatment protocols since the beginning of the post-World War II period. PTC has been treated with unilateral lobectomies [15] or subtotal/total thyroidectomies [16] with or without lymph node dissection, radioactive iodine therapy, and/or thyroid hormone suppression. A national cancer registry was used to update data specific to pediatric PTC in patients undergoing resection as well as to provide more insight into long-term outcomes and clinical characteristics in order to identify possible parameters defining the best surgical approach.
Methods
The surveillance, epidemiology, and end results (SEER) database April 2014 release was used to identify and analyze all cases of pediatric papillary thyroid carcinoma (PTC) in patients undergoing resection in the United States between 1973 and 2011. Cases were limited to children ≤20 years of age. Tumor histology in the SEER database was identified using the International Classification of Diseases for Oncology, 3rd revision. All variants of PTC, including follicular, were included in the database. Duplicate cases were not included in the sample.
SEER*Stat software, version 18.0 (National Cancer Institute; Bethesda, MD) was employed to obtain incidence and survival data. All data on incidence were age adjusted and normalized to the 2000 US standard population. Annual percentage change, in occurrence from baseline, was calculated using the weighted least-squares method supported and built into to the database. Statistical analyses were conducted using SPSS, version 21.0 (IBM; Armonk, NY). Categorical variables were compared using χ 2 or Fisher’s exact tests as appropriate. Continuous measures were compared using student’s t tests. Overall and disease-specific survival curves were calculated using the Kaplan–Meier method and comparisons were made using the log-rank test. All analyses were limited to available data.
The propensity score match was performed using the 1:1 nearest neighbor method. We have accounted for demographic (age group, gender race) and clinical (stage, number of lymph nodes) variables. For analysis by tumor size, procedure was added as a covariate; for analysis by procedure, tumor size was added as a covariate. Analyses were performed using the MatchIt supplement to the R statistical package (R project for statistical computing). Statistical significance was considered at P < 0.05.
Results
Patient demographics and tumor characteristics
A total of 2504 children and adolescents with pediatric PTC undergoing resection were identified during the study period. The overall age-adjusted population incidence rate was 0.483/100,000 per year (Table 1). This rate significantly increased by a 2.07 % annual percent change (APC) in occurrence from baseline (P < 0.05, Fig. 1). Incidence was highest in Caucasians, girls, adolescents (15–19 years old) and patients with regional disease (Table 2).
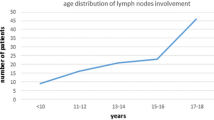
Patient demographics and clinical data are summarized in Table 2. Mean age of our cohort was 16 years of age (range 0–19 years, Fig. 2). Ratio of females to males was approximately 4.6:1 (n = 2096 versus 455, respectively). Most patients were between the ages of 15 and 19 (76.7 %) and 480 (19.2 %) patients were 10–14 years old. A total of 293 (14.4 %) patients with papillary thyroid carcinoma had tumors <1 cm while tumors ≥1 cm were found in 1745 (85.6 %) cases. Over one-third of cases (n = 828; 40.6 %) had tumors greater than 2.5 cm. Similar to incidence rates of disease extent, most patients (51 %) had regional disease, and only a minority of patients (8 %) presented with distant metastases.
Information on the type of surgical intervention was available for most of the cohort (n = 2077; 82.9 %) and an overwhelming number (n = 1881; 90.6 %) of patients underwent either a near total or total thyroidectomy. Although 8 % (n = 174) of patients received a thyroid lobectomy and/or isthmusectomy, less than 2 % (n = 22) had partial lobectomy. The proportion of subtotal/total thyroidectomies and lobectomies and/or isthmusectomies performed were similar in the 1991–2000 (88.6 and 8.8 %, respectively) and 2001–2011 (89.7 and 7.7 %, respectively) decades. Comparable proportions were observed in the 1980′s, however only 75 total cases were reported.
Available data on 523 patients pertaining to tumor grading demonstrated that 74.6 % of patients had well-differentiated tumors. Less than a quarter were poorly to moderately differentiated PTC.
Patients whose tumor sizes were <1 cm were more likely to receive thyroid lobectomies and/or isthmusectomies versus subtotal/total thyroidectomies [OR = 3.03 (2.12, 4.32), P < 0.001]; 34.4 versus 14.8 %, respectively). Subtotal/total thyroidectomy patients were more likely to have distant metastasis (including, but not limited to, the lung, liver and bone) [OR = 2.91 (0.70, 12.2); P < 0.05] versus regional disease (tumor extending beyond thyroid gland and/or invading neck lymph nodes) (8.8 and 54.1 %, respectively), when compared to thyroid lobectomy and/or isthmusectomy patients (1.2 and 21.1 %).
The majority of adjuvant or radiation-treated patients received radioisotope therapy (53 %) and the minority underwent external beam radiation (1.7 %) and radioactive implantation (1.2 %). Adjuvant therapy [OR = 8.00 (5.49, 11.66); P < 0.0001] was associated with receiving a subtotal and/or total thyroidectomy (68.8 %) versus thyroid lobectomy and/or isthmusectomy (21.6 %) (see Table 2). Specifically, while the majority of patients who underwent lobectomies and/or isthmusectomies (78.8 %) did not receive adjuvant therapy, over 60 % of subtotal/total thyroidectomy patients did receive some type of radiation therapy (P < 0.05). Approximately 65 % of these patients received radioactive iodine. Of the patients that did not undergo adjuvant treatment, most were subtotal/total thyroidectomy patients (78.9 %) and the minority were lobectomy and/or isthmusectomy patients (18.5 %) (P < 0.05).
With regards to lymph node dissection, subtotal and/or total thyroidectomy patients tended to have > 10 lymph nodes sampled (OR = 9.76 [4.29, 22.19]; P < 0.0001) compared to those who underwent thyroid lobectomies and/or isthmusectomies (26.7 vs. 3.6 %, respectively). Patients with tumors ≥1 cm had a higher lymph node ratio (range 3.4–10), or number of positive lymph nodes out of total amount of lymph nodes dissected, compared to those with tumors <1 cm (range 0.1–3.3) [OR = 2.55 (1.6, 4.1); P = 0.0002].
Propensity score matching was used to analyze tumor size and procedure type. When analyzed by tumor size, larger tumors (≥1 cm) tended to be lymph node positive [OR = 39.4 (16.6, 93.7), P < 0.001; 96.6 %] compared to tumors <1 cm (42.1 %). When propensity score was matched by procedure type, patients who underwent subtotal/total thyroidectomies had larger tumors ≥1 cm (100 %) and lymph node positive statuses (98.0 %) [OR = 99.0 (12.5, 783); P < 0.001] compared to thyroid lobectomies and/or isthmusectomies (73.5 and 32.7 %, respectively).
Survival
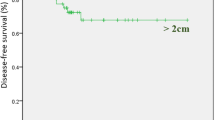
Survival measures according to clinical characteristics are displayed in Table 3. Disease-specific mean survival was 38.6 years for the whole cohort. Females had similar survival compared to males. Those with localized disease (limited to the thyroid gland) had better survival than patients with regional (tumor extending beyond thyroid gland and/or invading neck lymph nodes) or distant disease (P < 0.05) (Fig. 3). Of note, survival did not differ based on procedure type. Regardless of the type of surgery, disease-specific 5-, 15-, and 30-year survivals were maintained at 100 %. Neither tumor size nor performance of lymphadenectomy was associated with survival.
Discussion
The SEER program of the National Cancer Institute (NCI) is the largest cancer registry and a major source of incidence and survival data in the United States. Information is gathered from 20 population-based registries, covering approximately 28 % of the US population, in order to provide perspective on cancers on the national level. It is one of the few comprehensive sources of population-based information in the United States to include stage of cancer at the time of diagnosis and patient survival data. The NCI, in collaboration with the North American Association of Central Cancer Registries, guide all state registries to achieve data compatibility acceptable for pooling data and improving national estimates. A wide array of pediatric malignancies has been described using the SEER database [4, 17–25].
PTC, including its follicular variant, accounts for over 90 % of pediatric thyroid cancer [5], and its low incidence of 0.483/100,000 per year in this study’s cohort is consistent with previous reports [26, 27]. Nevertheless, this rate has been increasing by 2.07 % per year since the beginning of this study period. Since a long latency period between radiation exposure and disease exists, this increased rate is possibly, in part, due to an increased exposure to ionizing radiation. Prior studies have confirmed a clear association between ionizing radiation and childhood thyroid cancers [28–30].
Previously published data corroborate our study which demonstrates a growing incidence with increased age and a peak incidence in the 15–19 age group [31]. Caucasians had a higher incidence in this current analysis, consistent with other studies on PTC demographic data [32]. The predominance of PTC in white adolescent females has been a persistent finding in the literature [1, 5, 33, 34]. In our cohort, the ratio of adolescent females to adolescent males with PTC was almost 4.5:1. Our adolescent female to male ratio was higher than other studies and it can likely be attributed to the higher proportion of females in our dataset (81.7 %). A common explanation for the large proportion of PTC in females has been linked to the correlation between sex hormone changes during puberty and/or pregnancy and the increased risk of thyroid cancer [13].
Optimal surgical therapy of PTC in children and adolescents remains controversial since risks may outweigh benefits when considering its indolent nature and low mortality rates. Furthermore, the lack of prospective randomized clinical trials, partially owing to PTC’s rarity, has contributed to this effect [2]. Patients in the cohort who received a subtotal/total thyroidectomy were more likely to have distant disease, >10 lymph nodes sampled, and adjuvant treatment with radiation therapy. This is in parallel with a retrospective study of a multi-institutional cohort by Newman et al., which recommends that patients with more extensive disease should undergo a total thyroidectomy [32]. Correspondingly, it has been advocated to perform a total or subtotal thyroidectomy in patients with distant metastases found clinically or on preoperative imaging [11]. In this current study, patients with tumor sizes <1 cm were three times more likely to receive thyroid lobectomies and/or isthmusectomies compared to subtotal or total thyroidectomies. Thompson et al. suggested that in tumors less than 1 cm it might be appropriate to perform thyroid lobectomies alone [29]. Others have suggested this more conservative approach [1, 16, 35] regardless of tumor size because of the high risk of postoperative complications such as recurrent laryngeal nerve injury and permanent hypoparathyroidism [36, 37].
Still, many surgeons are proponents of a subtotal or total thyroidectomy [6, 13, 29] as it provides several advantages: (1) thyroglobulin levels are more useful markers of relapse, (2) the existence of residual microcarcinoma in the contralateral lobe can potentially lead to recurrence or transformation into a lethal histologic type, (3) it allows for a more efficient use of postoperative radioactive iodine, and (4) total body scintigraphy can be performed to find possible metastases [10]. Hay and associates studied 215 pediatric patients with PTC and showed a 6 % local recurrence rate at 40 years in the bilateral lobar resection group compared to a significantly higher 35 % rate in patients who received a unilobar resection as the initial surgical treatment [5]. Indeed, several studies have shown a higher risk of local recurrence in children who received lobectomies versus total or near-total thyroidectomies [38–40]. This is partially attributable to the fact that at least 40 % of PTCs are multifocal and bilateral at time of diagnosis [2]. Unfortunately, this study database lacks information on recurrence and cannot provide corresponding analyses.
Survival did not differ based on procedure type and disease-specific 5-, 15-, and 30-year survival rates remained 100 % in all surgical patients. This parallels a recent study by Sosa and colleagues on PTC in the adult population [41]. Accordingly, in a case series of 28 patients, Massimino and associates found the progression-free and overall survivals to be independent of type of surgery [35].
Because of limited data in the pediatric population, the extent of lymph node dissection is debatable. More aggressive lymph node dissections have been discouraged due to the potential for increased postoperative morbidity. Different treatment strategies including routine removal of jugular-carotid chain lymph nodes, sentinel node resection, and “berry picking” have been employed. In this current analysis, patients who underwent a subtotal or total thyroidectomy had tumor sizes ≥1 cm and positive lymph nodes statuses. Importantly, those who had tumors ≥1 cm were 39 times more likely to have positive lymph nodes. It is known that metastatic spread to lymph nodes, specifically cervical, increases recurrence rates [42]. Also, metastatic spread to locoregional lymph nodes has been identified as one of the most important risk factors for distant spread [6]. Demidchick and Kontratovich showed that children who had an initial lymph node dissection bilaterally had a lower risk of requiring repeat surgery [43]. Yet, several studies as well as the guideline from the National Comprehensive Cancer Network recommend central compartment lymph node resection (CCLND) based on positive clinical and/or radiographic evidence [8, 44]. This current study did not show any differences in survival with regards to lymph node status. This is in agreement with prior studies found in the literature that report lymph node involvement to be associated with recurrence but not survival [12, 32, 34]. These findings may further support the recommendation to avoid routine lymph node sampling and reserve CCLND to patients with clinically or radiographically detected lymph node disease. Nevertheless, we did find patients with tumors ≥1 cm to have had a higher lymph node ratio dissection. Unfortunately, SEER does not provide recurrence data and therefore we were not able to correlate lymph node ratio with recurrence. However, Schenider et al. demonstrated that elevated lymph node ratio was significantly associated with recurrence in adult PTC [45].
In this study, children with PTC had an excellent prognosis with disease-specific survival ranging from 99 to 100 %, akin to rates in the literature, supporting the conclusion that death from pediatric PTC over long follow-up periods is rare [1, 11]. In fact, survival rates in pediatric PTC were so high that the median survival had not been reached during this study period and therefore cannot be reported. Of note, however, the presence of distant metastasis conferred a lower survival in patients compared to local or regional disease as seen in a case series by Landau et al. [46]. Conversely, the presence of pulmonary metastases at diagnosis or within 6 months from initial presentation in another review [16] found no deaths after median follow up of 27 years with 100 % 5- and 10-year survival rates.
Although SEER is excellent in providing clinical and survival characteristics of various malignancies, it still has limitations to take into account. Cancer-related chemotherapy and radiotherapy data is incomplete within the database and lacks details. Similarly, information on the type of lymph node dissection or the use of diagnostic imaging to guide dissection does not exist in the database. The results found in SEER also may be skewed by some areas and ethnicities being over or underrepresented in the data set. Of importance to our review and as previously stated, SEER does not contain data on recurrence and unfortunately we cannot relate this lacking data to other studies. Nevertheless, the database remains a comprehensive resource in providing cancer-related outcomes and in our review reflects that if tumor size is ≥1 cm, lymph nodes will more likely be positive. Other studies have shown that there is an increased risk of recurrence when thyroid lobectomies are performed compared to subtotal/total thyroidectomies and that although metastasis to lymph nodes does not alter survival, it has been associated with increased recurrence. Therefore, although lymphadenectomy or procedure type does not alter survival, the initial surgical approach may have an effect on overall recurrences and a subtotal/total thyroidectomy, and lymph node dissection based on clinical findings, may prevent the need for future surgical and/or nonsurgical interventions, thereby improving patient quality of life.
Conclusions
Despite the strong propensity for PTC in children and adolescents to present at an advanced stage with more recurrent disease and distant metastasis compared to the adult population, its overall survival and prognosis have been shown to be excellent. Of note, the incidence of pediatric papillary thyroid cancer is increasing and females have a higher incidence, but similar survival to males. In addition, tumors ≥1 cm were likely to be associated with positive lymph node status and although they were more likely to be resected by subtotal/total thyroidectomy as opposed to lobectomy, survival was high. Therefore, procedure type did not affect survival rates according to the SEER data.
References
Collini P, Massimino M, Leite SF et al (2006) Papillary thyroid carcinoma of childhood and adolescence: a 30-year experience at the istituto nazionale tumori in milan. Pediatr Blood Cancer 46:300–306
Dinauer CA, Breuer C, Rivkees SA (2008) Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol 20:59–65
Luster M, Lassmann M, Freudenberg LS et al (2007) Thyroid cancer in childhood: management strategy, including dosimetry and long-term results. Hormones 6:269–278
Hogan AR, Zhuge Y, Perez EA et al (2009) Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res 156:167–172
Hay ID, Gonzalez-Losada T, Reinalda MS et al (2010) Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg 34:1192–1202
Dzepina D (2012) Surgical and pathological characteristics of papillary thyroid cancer in children and adolescents. Int J Pediatr 2012:125389
Grigsby PW, Gal-or A, Michalski JM et al (2002) Childhood and adolescent thyroid carcinoma. Cancer 95:724–729
Diesen DL, Skinner MA (2012) Pediatric thyroid cancer. Semin Pediatr Surg 21:44–50
Zimmerman D, Hay ID, Gough IR et al (1988) Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 104:1157–1166
Spinelli C, Bertocchini A, Antonelli A et al (2004) Surgical therapy of the thyroid papillary carcinoma in children: experience with 56 patients < or = 16 years old. J Pediatr Surg 39:1500–1505
La Quaglia MP, Black T, Holcomb GW 3rd et al (2000) Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the surgical discipline committee of the children’s cancer group. J Pediatr Surg 35:955–959 (discussion 960)
Cady B (1998) Presidential address: beyond risk groups—a new look at differentiated thyroid cancer. Surgery 124:947–957
Bal CS, Padhy AK, Kumar A (2001) Clinical features of differentiated thyroid carcinoma in children and adolescents from a sub-himalayan iodine-deficient endemic zone. Nucl Med Commun 22:881–887
Schlumberger M, Tubiana M, De Vathaire F et al (1986) Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab 63:960–967
La Quaglia MP, Corbally MT, Heller G et al (1988) Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery 104:1149–1156
Brink JS, van Heerden JA, McIver B et al (2000) Papillary thyroid cancer with pulmonary metastases in children: long-term prognosis. Surgery 128:881–886 (discussion 886-887)
Perez EA, Gutierrez JC, Koniaris LG et al (2009) Malignant pancreatic tumors: incidence and outcome in 58 pediatric patients. J Pediatr Surg 44:197–203
McAteer JP, Huaco JA, Gow KW (2013) Predictors of survival in pediatric adrenocortical carcinoma: a surveillance, epidemiology, and end results (seer) program study. J Pediatr Surg 48:1025–1031
Gutierrez JC, Fischer AC, Sola JE et al (2007) Markedly improving survival of neuroblastoma: a 30-year analysis of 1,646 patients. Pediatr Surg Int 23:637–646
Yang R, Cheung MC, Zhuge Y et al (2010) Primary solid tumors of the colon and rectum in the pediatric patient: a review of 270 cases. J Surg Res 161:209–216
Allan BJ, Wang B, Davis JS et al (2014) A review of 218 pediatric cases of hepatocellular carcinoma. J Pediatr Surg 49:166–171
Allan BJ, Parikh PP, Diaz S et al (2013) Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (Oxford). 15:741–746
Allan BJ, Thorson CM, Davis JS et al (2013) An analysis of 73 cases of pediatric malignant tumors of the thymus. J Surg Res 184:397–403
Davis JS, Allan BJ, Perez EA et al (2013) Primary pediatric cardiac malignancies: the seer experience. Pediatr Surg Int 29:425–429
Kassira N, Pedroso FE, Cheung MC et al (2011) Primary gastrointestinal tract lymphoma in the pediatric patient: review of 265 patients from the seer registry. J Pediatr Surg 46:1956–1964
Young JL Jr, Percy CL, Asire AJ et al (1981) Cancer incidence and mortality in the United States, 1973–77. Natl Cancer Inst Monogr 57:1–187
Harness JK (1997) Childhood thyroid carcinoma. In: Clark OH, Duh Q-Y (eds) Textbook of endocrine surgery. Saunders, Philadelphia pp 75–81
Tucker MA, Jones PH, Boice JD Jr et al (1991) Therapeutic radiation at a young age is linked to secondary thyroid cancer. The late effects study group. Cancer Res 51:2885–2888
Thompson GB, Hay ID (2004) Current strategies for surgical management and adjuvant treatment of childhood papillary thyroid carcinoma. World J Surg 28:1187–1198
Wiersinga WM (2007) Management of thyroid nodules in children and adolescents. Hormones 6:194–199
Harach HR, Williams ED (1995) Childhood thyroid cancer in england and wales. Br J Cancer 72:777–783
Newman KD, Black T, Heller G et al (1998) Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the surgical discipline committee of the children’s cancer group. Ann Surg 227:533–541
Collini P, Mattavelli F, Pellegrinelli A et al (2006) Papillary carcinoma of the thyroid gland of childhood and adolescence: morphologic subtypes, biologic behavior and prognosis: a clinicopathologic study of 42 sporadic cases treated at a single institution during a 30-year period. Am J Surg Pathol 30:1420–1426
Mazzaferri EL, Jhiang SM (1994) Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428
Massimino M, Collini P, Leite SF et al (2006) Conservative surgical approach for thyroid and lymph-node involvement in papillary thyroid carcinoma of childhood and adolescence. Pediatr Blood Cancer 46:307–313
Farrar WB, Cooperman M, James AG (1980) Surgical management of papillary and follicular carcinoma of the thyroid. Ann Surg 192:701–704
Gemsenjager E, Heitz PU, Martina B (1997) Selective treatment of differentiated thyroid carcinoma. World J Surg 21:546–551
Hay ID, McConahey WM, Goellner JR (2002) Managing patients with papillary thyroid carcinoma: insights gained from the mayo clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc 113:241–260
Hay ID, Bergstralh EJ, Goellner JR et al (1993) Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 114:1050–1057
Hay ID, Thompson GB, Grant CS et al (2002) Papillary thyroid carcinoma managed at the mayo clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885
Adam MA, Pura J, Gu L et al (2014) Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg 260:601–605
Nemec JZV, Pohunkova D et al (1986) Some factors influencing the survival of patients with less advanced stages of differentiated thyroid cancer. Endocrinol Exp. 20:85–95
Demidchik Iu E, Kontratovich VA (2003) Repeat surgery for recurrent thyroid cancer in children. Vopr Onkol 49:366–369
Chan AC, Lang BH, Wong KP (2013) The pros and cons of routine central compartment neck dissection for clinically nodal negative (cn0) papillary thyroid cancer. Gland Surg 2:186–195
Schneider DF, Mazeh H, Chen H et al (2013) Lymph node ratio predicts recurrence in papillary thyroid cancer. Oncologist 18:157–162
Landau D, Vini L, A’Hern R et al (2000) Thyroid cancer in children: the royal marsden hospital experience. Eur J Cancer 36:214–220
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Author contributions
JES, JIL, ARH, and EAP contributed to study conception and design. SG, JT, and EAP contributed to acquisition, analysis and interpretation of data. SG, JT, JES, ARH, and EAP contributed to drafting of manuscript. All authors contributed to critical revision of manuscript.
Financial disclosures
No authors have any financial affiliations to disclose.
Rights and permissions
About this article
Cite this article
Golpanian, S., Perez, E.A., Tashiro, J. et al. Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int 32, 201–208 (2016). https://doi.org/10.1007/s00383-015-3855-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3855-0