Abstract
Purpose
Experience with male cloaca (MC), a single opening in perineum for passage of urine and meconeum is described.
Methods
Cases of MC were ambispectively studied, prospectively from July 2007 to April 2015 and retrospectively for last three decades.
Results
Seven cases of MC were identified, between the ages of newborn—4 years (median 10 days). Two missed cases underwent a colostomy, posterior sagittal anorectoplasty, and urethroplasty. Two cases underwent perineal urethrostomy and anoplasty followed by urethroplasty. In one case, part of the rectal wall was used to form urethral tube and urethrostomy. For three recent cases, posterior sagittal anorectourethroplasty was done with mobilization of rectal pouch and common channel, separation of common wall between the urethra and rectum, urethroplasty varying from 1.5 to 3 cm, perineal body reconstruction, perineal urethrostomy and anoplasty. Follow-up of 6 patients varied from 3 months to 23 years. One case is lost to follow-up. Three patients have completed repair. Complications included a discharging sinus and a urethral fistula in one case each. One patient died while awaiting urethroplasty. Two patients are awaiting formal urethroplasty.
Conclusion
With familiarity of varying anatomy of MC, early recognition can avoid a neonatal colostomy in selected patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cloaca is a term commonly used for a posterior opening that serves as the only opening for the digestive, reproductive, and urinary tracts of certain animal species like amphibians, reptiles, birds and egg-laying mammals (monotremes). On the other hand, most placental mammals including humans possess two or three separate orifices for evacuation. The human embryonic cloaca divides into a posterior region that becomes part of the anus, and an anterior region that has different fates depending on the sex of the individual: in females, it develops into the vestibule that receives the urethra and vagina, while in males, it forms the penile urethra. Congenital disorders commonly described with a cloacal anomaly have been persistent cloaca, commonly called as common cloaca seen in females and the mermaid syndrome (sirenomelia). Persistent cloaca in females has been adequately studied and frequently reported in literature [1, 2]. Pena in 1998, described posterior cloaca as a unique defect in which urethra and vagina are fused together forming a urogenital sinus that deviates posteriorly, and opens in the anterior rectal wall at the anus or immediately anterior to it [3]. We describe here our experience with the counterpart of a cloacal anomaly in males.
Purpose
Male cloaca, a rare anomaly, is being identified more often after familiarity with the lesion. Changing trends in management with improving learning experience are described.
Methods
Cases of male cloaca were prospectively identified and studied from July 2007 to April 2015. A retrospective search was made for missed cases of male cloaca tackled over last three decades. Male cloaca was defined as a single opening in perineum in a male child through which the baby passed urine and meconeum or stool. The length of the common channel was noted from records in retrospective cases and measured with a plastic scale during surgery in prospective cases. The anorectal anomaly was classified as high (> or = 2 cm) or low (< 2 cm) depending on the craniocaudal distance of the separate anorectal opening above the common channel from the neo anus with 2 cm as the cutoff. The common channel opening was classified as wide if more than or equal to 1 cm, and narrow if less than 1 cm in diameter.
Results
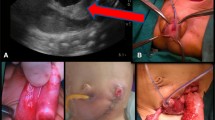
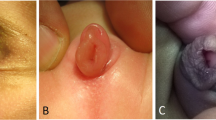
Seven cases of male cloaca were identified including 2 as missed diagnosis. The age group was newborn—4 years (median 10 days) (Table 1). The abdomen was not distended in any of the cases. The location of the cloacal opening was in the perineum. Contrast enema study when performed through the opening in one case delineated the bladder anteriorly once, and the large bowel posteriorly, when repeated (Fig. 1). On careful examination, two openings could be identified inside the single opening in two babies (Fig. 2). There was associated chordee in 6 cases, hypoplastic urethra in one and vaginal pouch in one. The characteristics of the common channel in one of the missed cases could not be documented from records. Clinical details of the second missed case were available. The length of the common channel varied from 1 to 3 cm (mean 2 cm). The opening was wide:narrow in 4:2 cases. Depending on the craniocaudal distance of the separate anorectal opening above the common channel from the neo anus, the anomaly was high:low in 4:2 cases. Cases 1 and 2, with a missed diagnosis, underwent a colostomy, posterior sagittal anorectoplasty, and urethroplasty. Cases 3–7 were managed without a colostomy. Case 3 underwent perineal urethrostomy and anoplasty at birth and later urethroplasty, followed by urethral fisula closure, perineal body reconstruction and anoplasty. For case 4, a part of the rectal wall was used to form the urethral tube and position the perineal urethrostomy at a distance from the neoanus. For cases 5–7, posterior sagittal anorectourethroplasty was done with mobilization of the rectal pouch and the common channel, meticulous separation of the common wall between the urethra and rectum, urethroplasty varying from 1.5 to 3 cm, perineal body reconstruction, perineal urethrostomy and anoplasty (Fig. 3). The duration of follow-up of 6 patients varies from 3 months to 23 years. Case 4 is lost to follow-up. Three patients have undergone complete repair. Complications included a discharging sinus in case 1, managed conservatively for 20 years and recently excised, and a urethral fistula in case 3. One patient died while awaiting urethroplasty due to a related cause. Two patients are still awaiting formal urethroplasty. Five patients assessed for continence as per the Krickenbecks criteria have voluntary bowel movements with no soiling and no constipation, though one patient is only 14 months in age and too young to assess continence. Continence could not be assessed in two patients, one was lost to follow up and there was one mortality.
Operative photographs depicting the steps of Posterior sagittal anorectourethroplasty (PSARUP). a Stay sutures are put around the common channel including the two openings within depicting the posterior rectal fistula and the anterior urethral opening. b A midline posterior sagittal incision is given c mobilization of the rectum is done posteriorly and laterally. d Gentle dissection is done to separate the anterior wall of the rectum from the urethra, creating two walls out of one The rectum is further mobilized all around till there is adequate mobility to place it within the sphincter complex e urethroplasty is done using the common wall to form the urethral lumen and construct the urethrostomy in the perineum. Perineal body is reconstructed behind the urethra. f Rectum is placed and sutured within the sphincter complex and anoplasty is done
Operative procedure (Fig. 3)
Posterior sagittal anorectourethroplasty (PSARUP) is begun in the same way as PSARP in the prone position. Stay sutures are put all around the common channel including the urethra and rectum. A midline posterior sagittal incision is given. Mobilization of the rectum is done posteriorly and laterally. The length of the common channel is assessed. The common channel is opened posteriorly from caudal to cranial direction till two openings are identified. Stay sutures are applied on posterior opening of urethra that forms a common sheath between the urethra and rectum. Gentle dissection is done to separate the anterior wall of the rectum from the urethra, creating two walls out of one, as done for separating vagina from rectal pouch in females. The urethra is catheterized. The common channel is used to reconstruct the urethra by approximating its posterior wall and doing a urethroplasty. The rectum is further mobilized all around till there is adequate mobility to place it within the sphincter complex. The perineal body is reconstructed behind the urethra. Urethrostomy is constructed in the perineum. The rectum is placed and sutured within the sphincter complex and anoplasty is done.
Discussion
Cloacal septation occurs by the in growth of epithelium-covered mesenchymal folds, craniocaudal Tourneux fold, and lateral to medial right and left Rathke folds. These folds, creating the urorectal septum, ultimately join the cloacal membrane, yielding the primitive urogenital sinus and the anorectal canal as two separate tubes. By the early 7th week of human gestation, the urogenital sinus and the anorectal canal are separate entities. Differentiation of the external genitalia is not initiated until about the 8th week that coincides with the testicular or ovarian differentiation depending on the activation of the SRY gene. In males, in response to androgenic stimulation, the perineum and labioscrotal folds fuse in midline, and genital tubercle and urethral plate undergo elongation and masculinization through the 4th month of gestation, yielding an elongated tubularized urethra.
Male cloaca is a rare anomaly that can be described as representing inability of the urorectal septum to meet the cloacal membrane. Its association with defects in the development of the external genitalia can be explained with simultaneous timing of the development of the urorectal septum and the urethral tubularization. Chordee was associated in six of the seven cases described.
The first case of a cloacal anomaly in a male was reported in 1929 [4]. Other names used to describe the anomaly include agenesis of the cloacal membrane, partial urorectal septum malformation sequence, and urorectal septum malformation sequence [5, 6]. Wheeler et al. described the anomaly in 7 males out of a series of 25 babies [6]. Qureshi et al. reported cloacal anomaly in 8 male stillborn fetuses based on autopsy findings [7]. It seems the anomaly is associated with a high mortality if picked during the antenatal period [7].
We presented our initial findings in two cases (cases 3, 4) in 2012 [8]. Bano et al. reported four male patients with cloacal variants [9]. The next commonly related anomaly would be associated hypospadias and anorectal malformation. Sharma et al. described five cases with perineal-mound and genital-fold defects including three with imperforate anus with rectobulbar fistula and perineal hypospadias [10].
Interestingly, there are studies in mice that document the molecular basis of hypospadias and anorectal malformation [11, 12]. Cell surface molecules like B-subclass Eph and ephrin have been shown to play an important role in clinically significant midline cell–cell adhesion and fusion events like hypospadias and urorectal septum defects [11]. EphB2 and ephrin-B2 are coexpressed at the midline in fusing urethral/cloacal endoderm and underlying lateral mesoderm of the urorectal septum that migrates toward the caudal midline as the cloaca septates [11]. Genetically engineered mice mutant for the cell surface molecules, ephrin-B2 or EphB2; EphB3 manifest a variety of genitourinary and anorectal malformations [12].
With recent advances in antenatal diagnosis like 3D ultrasonography and fetal MRI, more and more cases of cloaca are likely to be picked up [13, 14]. Whereas previously, it was thought that a cloacal anomaly refers to a female child; the word should be used with caution as more cases of a cloacal anomaly in males that may be associated with a higher mortality and associated anomalies are recognized.
The cardinal features of a male cloaca include passage of meconeum stained urine, a single opening in the perineum, and a decompressed abdomen. The anal mucosa may project towards the perineum and merge with the urothelium. Cases with signs of a low anorectal malformation like a meconeum lined tract along with hypospadias should be examined carefully to identify the rectal/anal fistula and the urethral opening separately. There may be an associated vaginal pouch as seen in one of our cases and two cases reported by Bano et al.
The cases which we described had a single opening in the perineum in front of the normal location of the anus, severe hypospadias with chordee and associated bifid scrotum or penoscrotal transposition. One case had a urethral opening in the anterior wall of the rectum along with a hypoplastic urethral duplication. In the initial cases, a anoplasty and urethrostomy was sufficient to give an outlet to the rectum. Perhaps such kind of anomalies are not diagnosed in routine clinical practice and are either subjected to an anoplasty or a colostomy and later urethroplasty followed by PSARP and then colostomy closure as was done for our missed case 1.
The important characteristics of the male cloaca that are worthwhile to study while planning the operative treatment are its location, width of the opening, length of common channel, and distance of separate anorectal opening from neoanus. Cases with a wide opening with free passage of urine and meconeum may not require an urgent correction in the neonatal period. They may wait for an appropriate age and weight for treatment to perform a PSARUP. Recognition of a low and a high male cloaca affects the management strategy. The extent of mobilization in the high cases is more and may be difficult in the neonatal period. In low cases with a narrow common channel, the channel may be opened posteriorly and an anoplasty may be done in the newborn period.
Though we could get a contrast study done in only one case, a cystoscopy through the common channel to locate the site of the urethral opening in the older patients may add useful information. However, there is a risk of infecting the urinary system with bacteria from the gastrointestinal tract.
Hypospadias is the norm in these patients. If a surgeon who is experienced in both colorectal surgery and pediatric urology deals with the case, it is easier to recognize this anomaly and deal with the common wall between the urethra and the rectum. By sharing the experience with more number of cases, one can appreciate the spectrum of the anomaly, add to the existing knowledge, and plan the requisite individualized management.
Conclusion
Cloacal anomaly in males is an extremely rare anomaly with very little available literature on the appropriate diagnostic and management options. We share our experience here. With familiarity and early recognition, one can avoid a neonatal colostomy in selected patients who are decompressing well. Posterior sagittal anorectourethroplasty (PSARUP) may be proposed as the ideal way to manage these patients with or without a covering colostomy.
References
Livingston JC, Elicevik M, Breech L, Crombleholme TM, Peña A, Levitt MA (2012) Persistent cloaca: a 10-year review of prenatal diagnosis. J Ultrasound Med 31:403–407
Bischoff A, Levitt MA, Lim FY, Guimarães C, Peña A (2010) Prenatal diagnosis of cloacal malformations. Pediatr Surg Int 26:1071–1075
Peña A, Kessler O (1998) Posterior cloaca: a unique defect. J Pediatr Surg 33:407–412
Major SG (1929) Persistence of the cloaca: report of a case. Minn Med 12:96–97
Qureshi F, Jacques SM, Yaron Y, Kramer RL, Evans MI, Johnson MP (1998) Prenatal diagnosis of cloacal dysgenesis sequence: differential diagnosis from other forms of fetal obstructive uropathy. Fetal Diagn Ther 13:69–74
Wheeler PG, Weaver DD (2001) Partial urorectal septum malformation sequence: a report of 25 cases. Am J Med Genet 103:99–105
Qureshi F, Jacques SM (2012) Cloacal abnormalities in male fetuses. J Ultrasound Med. 2012(31):2046–2047 (author reply 2047–8)
Sharma S, Gupta DK (2012). Cloaca in a male; diagnosis on suspicion. Presented during the 19th Colorectal Club meeting. Rome, 11–12 June. 2012. http://www.colorectalclub2012.altervista.org/program.html
Banu T, Chowdhury TK, Hoque M, Rahman MA (2013) Cloacal malformation variants in male. Pediatr Surg Int 29:677–682
Sharma AK, Goel D, Kothari SK (1999) Perineal-mound defects. Pediatr Surg Int 15:227–229
Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M (2004) Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol 271:272–290
Yucel S, Dravis C, Garcia N, Henkemeyer M, Baker LA (2007) Hypospadias and anorectal malformations mediated by Eph/ephrin signaling. J Pediatr Urol 3:354–363
Dannull K, Sung J (2014) Cloacal dysgenesis diagnosis by prenatal ultrasound and MRI. Pediatr Radiol 44:230–233
Le Borgne H, Philippe HJ, Le Vaillant C (2011) Contribution of three-dimensional ultrasonography in depicting perineal features in cloacal malformation. Fetal Diagn Ther 30:239–240
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma , S., Gupta, D.K. Male cloaca malformation: rare variant of anorectal malformation. Pediatr Surg Int 31, 747–752 (2015). https://doi.org/10.1007/s00383-015-3738-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3738-4