Abstract
Purpose
Pediatric hydrocephalus is the most common cause of surgically treatable neurological disease in children. Controversies exist whether endoscopic third ventriculostomy (ETV) or cerebrospinal fluid (CSF) shunt placement is the most appropriate treatment for pediatric hydrocephalus. This study aimed to compare the risk of re-operation and death between the two procedures.
Methods
We performed a retrospective population-based cohort study and included patients younger than 20-years-old who underwent CSF shunt or ETV for hydrocephalus from the Taiwan National Health Insurance Research Database.
Results
A total of 3,555 pediatric patients from 2004 to 2017 were selected, including 2,340 (65.8%) patients that received CSF shunt placement and 1215 (34.2%) patients that underwent ETV. The incidence of all-cause death was 3.31 per 100 person-year for CSF shunt group and 2.52 per 100 person-year for ETV group, with an adjusted hazard ratio (HR) of 0.79 (95% confidence interval [CI] = 0.66–0.94, p = 0.009). The cumulative incidence competing risk for reoperation was 31.2% for the CSF shunt group and 26.4% for the ETV group, with an adjusted subdistribution HR of 0.82 (95% CI = 0.70–0.96, p = 0.015). Subgroup analysis showed that ETV was beneficial for hydrocephalus coexisting with brain or spinal tumor, central nervous system infection, and intracranial hemorrhage.
Conclusion
Our data indicates ETV is a better operative procedure for pediatric hydrocephalus when advanced surgical techniques and instruments are available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is one of the most common diseases involving the central nervous system for both children and adult. Cerebrospinal fluid (CSF) shunt procedures can normalize intracranial pressure and minimize possible related neuronal injury and other unfavorable consequences [1, 2]. Due to the capability of the peritoneum to absorb cerebrospinal fluid, ventriculoperitoneal (VP) shunt is the most frequently utilized procedure for hydrocephalus. However, some conditions, such as valve failure, catheter obstruction or migration, shunt disconnection or infection, may cause shunt failure [3]. Shunt malfunctions happen more frequently in children than in adults. Shunt failures occur in 14% of cases within the 1st month [4] and 40–50% within the 1st year after shunt placement [5, 6] for pediatric hydrocephalus. It could also result in the need for one or more revision surgeries, sometimes requiring urgent treatment, and even shunt-related death [7, 8].
Endoscopic third ventriculostomy (ETV) is well accepted for hydrocephalus due to improvements in endoscopy, better imaging, advanced surgical techniques, and instruments. ETV developed with the benefit of device freedom for growing children in the last two decades. However, some neurosurgeons hesitate to perform ETV and still consider CSF shunt as the most prominent treatment of choice for pediatric hydrocephalus due to equipment limitation, skill difficulty, and risk of large vessel injury with ETV [9]. While many studies have shown the complications of CSF shunting for pediatric hydrocephalus, there is still a knowledge gap of the advantages and risks between CSF shunt and ETV because studies directly comparing the long-term outcomes of shunts or ETVs are rare.
Therefore, the aim of this study is to better understand the effectiveness of ETV based on real-world data from the Taiwan National Health Insurance Research Database (NHIRD). Our goal was (1) to report reoperation rate and long-term mortality of patients who underwent ETV or CSF shunt for pediatric hydrocephalus, (2) to compare the effectiveness of the two surgical procedures, and (3) to examine factors, such as age or tumor status, related to reoperation and mortality occurring after the first procedure.
Materials and methods
Data source
The Joint Institutional Review Board at Taipei Medical University Hospital approved the present retrospective cohort study with a waiver of informed consent (code of approval: N202005071). All experiments were performed in accordance with relevant guidelines and regulations. Data was obtained from NHIRD which contains reimbursement claims data under the regulation of National Health Insurance (NHI). NHI in Taiwan provides comprehensive care services and it is mandatory for all citizens in Taiwan to join NHI, resulting in a nearly 99% coverage rate of the 23 million residents of Taiwan. The NHIRD is used strictly for research purposes, and all personal identification data are encrypted to protect the confidentiality of patients before being released to researchers. The data that support the findings of this study are available from Taiwan National Health Insurance Administration but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon request and with permission of Taiwan National Health Insurance Administration.
Study design and study cohort
This study is a retrospective population-based cohort study with two groups and patients who were less than 20 years old and first received a surgical procedure of CSF shunt or ETV for hydrocephalus between 2004 and 2017. We excluded patients whose medical records could not clearly show the type of surgery performed, and who received both shunt and ETV at the same admission, but the medical records could not clearly show which procedure was done first. Eligible patients were classified into a group of patients who received CSF shunt and a group of patients who received ETV. The date of admission was referred as the index date thereafter.
Study outcomes
The primary outcome was all-cause death which defined if a patient had a death record derived from the National Death Registry after the index date. The secondary outcome was reoperation which was defined as a patient having a new surgical procedure related to hydrocephalus, no matter ETV or CSF shunt, after discharge based on the reimbursement claims of NHIRD.
Covariates
Baseline characteristics were assessed at the time of inclusion. We considered patient’s gender, age, year of procedure and previous or coexisting disease conditions one year prior to the index date, including brain/spinal tumor, brain vascular lesions, congenital anomalies of nervous system, central nervous system (CNS) infection, intracranial hemorrhage, and development disorders. The disease diagnosis codes for baseline comorbidities are provided in Supplementary Table 1.
Statistical analysis
Since baseline characteristics between CSF shunts and ETV subjects differed, we used an inverse probability of treatment weighting (IPTW) matching to balance the difference between two groups. This approach is highly recommended in observational studies that intend to compare different treatment alternatives, allowing us to estimate the relative treatment effect with minimal bias on time-to-event. Baseline characteristics were analyzed using standardized mean difference (SMD) and SMD of > 0.1 indicates the presence of non-negligible differences between 2 groups. Cox proportional model was used to examine the risk of death. Competing risk model was used for reoperation since the risk of death might differ between two groups and thus, we treated death as a competing risk when comparing the likelihood of the patients receiving reoperation between two groups. All models were additionally adjusted for baseline covariates listed in Table 1. All patients were followed at least one year from the index date to the date of outcomes of interests or the end of observational period (December 31st, 2017). All analyses were performed using SAS/STAT 9.4 (SAS Institute Inc., Cary, North Carolina) and STATA 14 (Stata Corp LP, College Station, Texas). A p < 0.05 was considered significant.
Results
Among 4049 pediatric patients receiving a shunt or ETV for hydrocephalus between 2004 and 2017, a total of 3555 were eligible (aged under 20-year-old at the time of receiving surgical treatment) for the current study. These patients were separated into two cohorts depending on the treatment procedure: 2340 (65.8%) for CSF shunt and 1215 (34.2%) for ETV (Fig. 1).
Baseline characteristics are shown in Table 1. Before IPTW, patients who received ETV were older, fewer male, with age between 0 and 1, but with higher proportion of brain tumors when compared to those who received shunts. ETV was also performed more frequently in more recent years. In terms of previous or coexisting disease conditions, patients with ETV were more likely to have a history of malignant neoplasm of brain, and benign brain and CNS tumors. After IPTW, baseline characteristics were well-balanced between the two groups.
The cumulative incidence and relative risks of reoperation and death are shown in Tables 2 and 3. After IPTW, the cumulative incidence competing risk (CICR) for reoperation was 31.2% for patients receiving shunt and 26.4% for patients receiving ETV, with an adjusted subdistribution hazard ratio (SHR) of 0.82 (95% CI of 0.70–0.96, p = 0.015). In terms of mortality, the incidence of all-cause death was 3.31 per 100 person-year (PY) for patients receiving shunts and 2.52 per 100 PY for patients receiving ETV, respectively, with an adjusted hazard ratio (HR) of 0.79 (95% CI of 0.66–0.94, p = 0.009).
Figure 2A presents the cumulative incidence competing risk of reoperation and we found the incidence of reoperation was high in the beginning after the index date and became stable later. A similar trend was found for the cumulative incidence of all-cause death based on Kaplan–Meier estimation (Fig. 2B); moreover, the difference occurred in the first year after the index date.
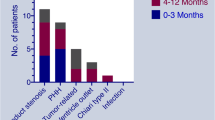
Subgroup analysis (Fig. 3) presents the difference between CSF shunt and ETV by age groups (age 0- to 9-year-old and 10- to 20-year-old), a history of brain or spine tumor and coexistence with CNS infection and intracranial hemorrhage. For reoperation, ETV benefits subgroups of the patients aged 0- to 9-year-old (HR = 0.74, 95% CI of 0.56–0.97) and subgroups coexisting with brain or spine tumor (reoperation; HR = 0.51, 95% CI of 0.33–0.78). However, the benefit effect of all-cause death only occurred in patients aged between 10- and 20-year-old (SHR = 0.55, CI of 0.38–0.81). In addition, ETV benefits both reoperation and all-cause-death for subgroups of hydrocephalus without CNS infection (reoperation: HR = 0.71, 95% CI of 0.57–0.90; all-cause-death: HR = 0.73, 95% CI of 0.56–0.96), and subgroups of hydrocephalus without intracranial hemorrhage (reoperation: HR = 0.66, 95% CI of 0.52–0.83; all-cause-death: HR = 0.70, 95% CI of 0.53–0.93).
Discussion
This study represents a significant contribution as the first large-scale population-based investigation demonstrating the efficacy of ETV and CSF shunt in pediatric patients with hydrocephalus. Compared to CSF shunt placement, ETV exhibits a relatively lower risk of all-cause death and reoperation. Despite ongoing debate regarding the optimal treatment for pediatric hydrocephalus, VP shunt placement has conventionally been the preferred approach among neurosurgeons in recent decades. There are still risks of complications after shunt procedure despite several revolutions and technical modifications. In contrast, ETV is more often considered for obstructive hydrocephalus in children older than 2 years and adults in recent years [10] because of the advantage of being device free and avoidance of shunt-related complication, such as shunt infection. This study used real-world data to show the benefit of ETV for pediatric hydrocephalus, showing ETV could be a treatment option for children in terms of mortality and reoperation compared to the more traditional shunt procedures.
Although placement of a CSF shunt is a common solution and a standard procedure for hydrocephalus [11], there are still problems after this operation, especially for the growing pediatric patients. The complications due to VP shunt have been reported in many studies, ranging from 20.0 to 84.5% [1, 12, 13]. Specifically, CSF shunts for pediatric hydrocephalus had a 33.2–50% failure rate [14, 15]; the incidence of shunt revision was 27–84.5%, shunt blockade 5–50%, shunt infection 9–23%, shunt migration or disconnection 3.6–11%, and shunt malfunction due to abdominal pseudocyst 10.8%. Hydrocephalus associated with post-tubercular meningitis and intraventricular hemorrhage in shunt placement had higher risk (35.13%, 45.4% respectively) with multiple shunt revisions [12]. 12.5% of patients were reported to need the first revision more than 10 years after initial shunt placement [1]. The shunt-related mortality reported was 5–13.7% [12, 16, 17].
Other treatments should also be considered due to the high incidence of the complications of CSF shunt placement. In the last two decades, CSF shunt treatment has trended toward endoscopic treatment for pediatric hydrocephalus [18,19,20]. Endoscopic procedures are usually described as minimally invasive and thought to have lower morbidity and mortality rates when compared to traditional or open microsurgical procedures [21, 22]. Moreover, ETV is considered preferable to CSF shunt procedures in pediatric patients more than 6-months-old, since it is at least as efficient as the shunt procedure, and it avoids shunt dependency and associated complications [23,24,25]. Despite the pros ETV offers, including being a shunt device free for pediatric hydrocephalus, there are risks of complications and failure, which may result in reoperation or even death. Rates of ETV failure are about 20–37%; variation depends on the time duration of postoperative follow-up [26, 27]. In 2010, Kulkarni et al. reported that the relative risk of ETV failure is initially higher than that for shunt, but after about 3 months, the relative risk becomes progressively lower for ETV, based on three international collaborative studies [26]. The overall complication rate of ETV is reported to be 5–15% [24, 28], and include bleeding from the walls of the lateral or third ventricle, larger vessels injury, like branches from basilar artery or superior cerebellar artery which can lead to severe morbidity [29]. Abhaya et al. reported 6.0% moderate or severe intraoperative bleeding during ETV procedure, while Bouras and Sgouros reported 3.7% considerable intraoperative bleeding [24, 25]. Meningitis or wound infection after ETV happened in 1.5–2% of procedures [24, 25], yet operation-related infection after ETV is less than after shunt procedure. However, some complications happen more after ETV, including CSF leakage (4.4%), hyponatremia (3.9%), pseudomeningocele (3.9%), seizure (2.0%), and new neurologic deficit (0.5–1.0%) [25].
In 2010, ETV Success Score (ETVSS) was developed to predict the success rate of ETV for pediatric hydrocephalus, based on age, etiology of hydrocephalus, and presence of a previous shunt [30]. A high ETVSS predicts a high chance of early ETV success. One of limitations of the present study, we could not get the ETVSS of every case from the NHIRD. Therefore, we used the age and different etiology of hydrocephalus (two variants of ETVSS) to analyze the risk of the reoperation rate and all-cause death. Age is a determining factor of success for both ETV and shunt procedure for pediatric hydrocephalus. Tuli et al. found both etiology and age less than 1 year at first shunt placement were risk factors for CSF shunt failure [6]. Two papers revealed that only age (< 6 months) and not etiology was a determinant of CSF shunt survival [31, 32]. Kulkarni et al. have reported an increased frequency of infection after shunt in children less than 6 months of age as compared to older than 1 year [33]. The success rate of ETV was also higher in children over 1-year-old in a population-based study [34]. Because there is no need for any device implantation in ETV procedure, the risk of long-term complications is reduced [20]. In our study, the case number of patients aged below 1-year-old is too small to analyze, so we separate all cases into 2 groups (patients aged from 0- to 9-year-old and 10- to 20-year-old). We found ETV had more benefit effect for both all-cause death and reoperation in patients aged from 10- to 20-year-old. The etiology of hydrocephalus is also an important factor of success of ETV. Tumor-associated hydrocephalus is common in primary pediatric brain tumors. In this context, ETV was found to have a high success rate of more than 90% and has been recommended as the ideal treatment for hydrocephalus in cases who underwent posterior fossa tumor removal surgery [35]. TT Wong reported 56.7% of pediatric brain tumor cases presented hydrocephalus, the hydrocephalus was obstructive type (98%) and rarely communicating type (1.9%) at tumor diagnosis[36]. ETV is recommended as the choice of treatment for obstructive hydrocephalus in many institutes now [29]. ETV was reported to provide 81.5% overall rate of shunt independence with clinical remission or improvement for obstructive hydrocephalus [37]. Our data also showed ETV had more benefit effect for reoperation in patients with a history of brain or spinal tumor in the long-time follow-up (Fig. 3A). The cumulative incidence of reoperation, as represented by the CICR, demonstrated elevated rates for both ETV and CSF shunt procedures during the initial 0–15 years of the analysis period, with ETV showing a comparatively lower incidence. This pattern aligns with the observations reported by Kulkarni et al. in 2010 over a 4-year follow-up [26]. Similarly, a parallel trend was observed in the Kaplan–Meier curves for all-cause death, where distinctions between the ETV and CSF shunt cohorts emerged in the first year following the index date, favoring a lower incidence in the ETV cohort.
ETV is mainly reserved for obstructive hydrocephalus in patients who have normal or near normal cerebrospinal fluid absorptive capacity [38]. Therefore ETV is appropriate for treatment of pediatric hydrocephalus secondary to obstruction due to brain tumor, spinal tumor, aqueductal stenosis, myelomeningocele, infection-related hydrocephalus, and intraventricular hemorrhage [34]. But the indications of ETV have recently expanded to communicating types of hydrocephalus [39, 40] and the success of ETV in infants with hydrocephalus has been increased by the addition of choroid plexus cauterization [10].
In our study, we present national data from Taiwan of pediatric hydrocephalus cases with ETV or shunt procedures in children. According to our results, the incidence competing risk of reoperation for children receiving CSF shunt was 27.4%, higher than children receiving ETV (18.4%) (Table 2). The rate of all-cause death of children was higher for shunt compared to ETV (3.29% vs. 1.67%) procedures (Table 3). Along with improvements in endoscopy equipment and technologies, the need for shunt independence has led to advances in ETV techniques. The success rate of ETV is directly proportional to the experience of operating neurosurgeon [41]. We can find a trend of more neurosurgeons performing ETV to treat pediatric hydrocephalus year by year in Taiwan. In Taiwan, there are few neurosurgeons subspecialized and well trained in pediatric neurosurgery and most neurosurgeons chose CSF shunt instead of ETV for pediatric hydrocephalus despite any etiology in the earlier period. Pediatric neurosurgeons’ training for ETV procedure for pediatric hydrocephalus may be one of the factors affecting the results of ETV or CSF shunt outcome in this data.
The strength of the current study is the large-scale data from a single database and long-term follow-up. However, we also recognize that our study has limitations. The largest limitation of this study is its retrospective nature being sourced from a national database with limited information. Hydrocephalus has been customarily classified as either obstructive or communicating and we included both types. Underlying etiology of hydrocephalus, like intraventricular hemorrhage, myelomeningocele and previous CSF infection were reported to be associated with failure of ETV or CSF shunt [42]. We could not classify the etiology of hydrocephalus which might influence the choice of ETV or CSF shunt procedure. In addition, the choice of treatment is influenced by the patients’ clinical end points. To minimize the confounding effect, statistical methods like multivariable regression, propensity score method, and instrumental variable-like methods are recommended to adjust the indications. However, none of these methods can completely resolve the effect of confounding through indications. In this study, we used multivariate regression models and patient subgroups to improve the validity of the findings. Lastly, CSF diversion procedures might vary in the success rate based on different types of shunts used. The claims data only included the reimbursement procedure of nonprogrammable VP shunt, limiting our analysis to only this information.
Conclusion
Currently, hydrocephalus is routinely treated and with great success. CSF shunt placement is still the main treatment for pediatric hydrocephalus, but ETV is becoming more common. CSF shunts and ETV procedures are both associated with different complications and morbidity. ETV procedure by experienced surgeons is an effective and safe tool in the management of pediatric hydrocephalus. Our study revealed ETV is superior to shunt for pediatric hydrocephalus aged below 20-year-old in success rate and long-time survival. However, underlying causative pathology and risk factor for ETV should still be studied in detail.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and supplementary material.
References
Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ (2013) Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr 11:15–19
Aoyama Y, Kinoshita Y, Yokota A, Hamada T (2006) Neuronal damage in hydrocephalus and its restoration by shunt insertion in experimental hydrocephalus: a study involving the neurofilament-immunostaining method. J Neurosurg 104:332–339
Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, Lafleur B, Dean JM, Kestle JR, Hydrocephalus Clinical Research N (2009) Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. Clinical article. J Neurosurg Pediatr 4:156–165
McGirt MJ, Leveque JC, Wellons JC 3rd, Villavicencio AT, Hopkins JS, Fuchs HE, George TM (2002) Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg 36:248–255
Liptak GS, McDonald JV (1985) Ventriculoperitoneal shunts in children: factors affecting shunt survival. Pediatr Neurosci 12:289–293
Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M (2000) Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg 92:31–38
Gmeiner M, Wagner H, Zacherl C, Polanski P, Auer C, van Ouwerkerk WJ, Holl K (2017) Long-term mortality rates in pediatric hydrocephalus-a retrospective single-center study. Childs Nerv Syst 33:101–109
Acakpo-Satchivi L, Shannon CN, Tubbs RS, Wellons JC 3rd, Blount JP, Iskandar BJ, Oakes WJ (2008) Death in shunted hydrocephalic children: a follow-up study. Childs Nerv Syst 24:197–201
Bhasin RR, Chen MK, Pincus DW (2007) Salvaging the “lost peritoneum” after ventriculoatrial shunt failures. Childs Nerv Syst 23:483–486
Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC (2016) Hydrocephalus in children. Lancet 387:788–799
Alcazar L, Alfaro R, Tamarit M, Gomez-Angulo JC, Ortega JM, Aragones P, Jerez P, Salazar F, del Pozo JM (2007) Delayed intracerebral hemorrhage after ventriculoperitoneal shunt insertion. Case report and literature review. Neurocirugia (Astur) 18:128–133
Pan P (2018) Outcome analysis of ventriculoperitoneal shunt surgery in pediatric hydrocephalus. J Pediatr Neurosci 13:176–181
Reddy GK, Bollam P, Caldito G (2014) Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg 81:404–410
Shprecher D, Schwalb J, Kurlan R (2008) Normal pressure hydrocephalus: diagnosis and treatment. Curr Neurol Neurosci Rep 8:371–376
Browd SR, Ragel BT, Gottfried ON, Kestle JR (2006) Failure of cerebrospinal fluid shunts: part I: Obstruction and mechanical failure. Pediatr Neurol 34:83–92
Tuli S, Tuli J, Drake J, Spears J (2004) Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg 100:442–446
Vinchon M, Baroncini M, Delestret I (2012) Adult outcome of pediatric hydrocephalus. Childs Nerv Syst 28:847–854
Isaacs AM, Bezchlibnyk YB, Yong H, Koshy D, Urbaneja G, Hader WJ, Hamilton MG (2016) Endoscopic third ventriculostomy for treatment of adult hydrocephalus: long-term follow-up of 163 patients. Neurosurg Focus 41:E3
Limbrick DD Jr, Baird LC, Klimo P Jr, Riva-Cambrin J, Flannery AM, Pediatric Hydrocephalus Systematic R, Evidence-Based Guidelines Task F (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: Cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J Neurosurg Pediatr 14 Suppl 1:30–34
Lotfinia I (2017) A review in pediatric hydrocephalus: physiology, classification, clinical presentation, imaging and treatment
Girgis F, Diaz R, Hader W, Hamilton M (2015) Comparison of intracranial neuroendoscopic procedures in children versus adults. Can J Neurol Sci 42:427–435
Sheikh AB, Mendelson ZS, Liu JK (2014) Endoscopic versus microsurgical resection of colloid cysts: a systematic review and meta-analysis of 1,278 patients. World Neurosurg 82:1187–1197
Soleman J, Guzman R (2020) Neurocognitive complications after ventricular neuroendoscopy: a systematic review. Behav Neurol 2020:2536319
Bouras T, Sgouros S (2011) Complications of endoscopic third ventriculostomy. J Neurosurg Pediatr 7:643–649
Kulkarni AV, Riva-Cambrin J, Holubkov R, Browd SR, Cochrane DD, Drake JM, Limbrick DD, Rozzelle CJ, Simon TD, Tamber MS, Wellons JC 3rd, Whitehead WE, Kestle JR, Hydrocephalus Clinical Research N (2016) Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr 18:423–429
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S, Canadian Pediatric Neurosurgery Study G (2010) Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67:588–593
Durnford AJ, Kirkham FJ, Mathad N, Sparrow OC (2011) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus: validation of a success score that predicts long-term outcome. J Neurosurg Pediatr 8:489–493
Bouras T, Sgouros S (2013) Complications of endoscopic third ventriculostomy. World Neurosurg 79(S22):e29–e12
Deopujari CE, Karmarkar VS, Shaikh ST (2017) Endoscopic third ventriculostomy: success and failure. J Korean Neurosurg Soc 60:306–314
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S, Canadian Pediatric Neurosurgery Study G (2010) Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr 6:310–315
Piatt JH Jr, Carlson CV (1993) A search for determinants of cerebrospinal fluid shunt survival: retrospective analysis of a 14-year institutional experience. Pediatr Neurosurg 19:233–241; discussion 242
Riva-Cambrin J, Kestle JR, Holubkov R, Butler J, Kulkarni AV, Drake J, Whitehead WE, Wellons JC 3rd, Shannon CN, Tamber MS, Limbrick DD Jr, Rozzelle C, Browd SR, Simon TD, Hydrocephalus Clinical Research N (2016) Risk factors for shunt malfunction in pediatric hydrocephalus: a multicenter prospective cohort study. J Neurosurg Pediatr 17:382–390
Kulkarni AV, Drake JM, Lamberti-Pasculli M (2001) Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94:195–201
Lam S, Harris D, Rocque BG, Ham SA (2014) Pediatric endoscopic third ventriculostomy: a population-based study. J Neurosurg Pediatr 14:455–464
Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C (2008) Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst 24:1405–1412
Wong TT, Liang ML, Chen HH, Chang FC (2011) Hydrocephalus with brain tumors in children. Childs Nerv Syst 27:1723–1734
Bouramas D, Paidakakos N, Sotiriou F, Kouzounias K, Sklavounou M, Gekas N (2012) Endoscopic third ventriculostomy in obstructive hydrocephalus: surgical technique and pitfalls. Acta Neurochir Suppl 113:135–139
Brichtova E, Chlachula M, Hrbac T, Lipina R (2013) Endoscopic third ventriculostomy in previously shunted children. Minim Invasive Surg 2013:584567
Hailong F, Guangfu H, Haibin T, Hong P, Yong C, Weidong L, Dongdong Z (2008) Endoscopic third ventriculostomy in the management of communicating hydrocephalus: a preliminary study. J Neurosurg 109:923–930
Rangel-Castilla L, Barber S, Zhang YJ (2012) The role of endoscopic third ventriculostomy in the treatment of communicating hydrocephalus. World Neurosurg 77:555–560
Egger D, Balmer B, Altermatt S, Meuli M (2010) Third ventriculostomy in a single pediatric surgical unit. Childs Nerv Syst 26:93–99
Duru S, Peiro JL, Oria M, Aydin E, Subasi C, Tuncer C, Rekate HL (2018) Successful endoscopic third ventriculostomy in children depends on age and etiology of hydrocephalus: outcome analysis in 51 pediatric patients. Childs Nerv Syst 34:1521–1528
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. Conceptualization: Shu-Mei Chen. Methodology: Shu-Mei Chen, Jiann-Her Lin, Tu-Hsueh Yeh, Wei-Lun Lo, Tai-Ngar Lui. Validation: Shu-Mei Chen, Yi-Chen Hsieh, Li-Nien Chien. Formal analysis: Li-Ying Chen. Investigation: Shu-Mei Chen, Li-Ying Chen. Data curation: Li-Ying Chen, Li-Nien Chien. Writing—original draft preparation: Shu-Mei Chen. Writing—review and editing: Nicole Salazar, Yi-Chen Hsieh, Li-Nien Chien. Project administration, Shu-Mei Chen.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, SM., Chen, LY., Lin, JH. et al. Comparison of endoscopic third ventriculostomy versus cerebrospinal fluid shunt procedures for the treatment of pediatric hydrocephalus in Taiwan. Childs Nerv Syst 40, 2883–2891 (2024). https://doi.org/10.1007/s00381-024-06469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-024-06469-7