Abstract
Purpose
To review a multicentric series of lateral-type posterior fossa ependymomas operated in the last ten years and to analyze the factors related to clinical evolution and tumor survival.
Methods
Descriptive, retrospective study. Active members of the Spanish Society of Pediatric Neurosurgery were invited to participate in this multicentric study. Clinical and radiological data were incorporated to an open database. The role of histologic grade, grade of resection, postoperative morbidities, and clinical follow-up was evaluated through bivariate associations (chi-square), Kaplan–Meier’s curves (log-rank test), and multivariate analysis (binary logistic regression).
Results
Fourteen centers entered the study, and 25 cases with a minimum follow-up of 6 months were included. There were 13 boys and 12 girls with a mean age close to 3 years. Mean tumor volume at diagnosis was over 60 cc. A complete resection was achieved in 8 patients and a near-total resection in 5 cases. Fifteen tumors were diagnosed as ependymoma grade 2 and ten as ependymoma grade 3. Major morbidity occurred postoperatively in 14 patients but was resolved in twelve within 6 months. There were six cases of death and 11 cases of tumor progression along the observation period. Mean follow-up was 44.8 months. Major morbidity was significantly associated with histologic grade but not with the degree of resection. Overall and progression-free survival were significantly associated with complete surgical resection. At the last follow-up, 16 patients carried a normal life, and three displayed a mild restriction according to Lansky’s scale.
Conclusions
Lateral-type posterior fossa ependymomas constitute a specific pathologic and clinical tumor subtype with bad prognosis. Gross total resection is the goal of surgical treatment, for it significantly improves prognosis with no additional morbidity. Neurological deficits associated to lower cranial nerve dysfunction are common, but most are transient. Deeper genetic characterization of these tumors may identify risk factors that guide new treatments and stratification of adjuvant therapies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central nervous system tumors constitute the most common tumors in pediatric patients, with an incidence of 5.74 cases per 100,000 pediatric population. Ependymomas are uncommon tumors which barely represent 5% of primary central nervous system tumors at this pediatric age [17]. Overall, survival of ependymomas exceeds 70% of cases after complete resection and adjuvant treatment [18, 22, 25]. However, some subtypes of ependymomas show a more unfavorable prognosis.
The latest WHO classification on central nervous system tumors differentiates ependymomas into nine distinct groups, taking into consideration anatomic, histopathologic, and genetic features [14]. Posterior fossa A ependymomas constitute a specific subtype of tumors with a distinct molecular signature characterized by EZHIP and H3K27 mutations and DNA hypermethylation. It is the group with the worst prognosis. Further genetic features may predict an even more aggressive behavior [1, 19, 20], but the correlation of these new findings with radiologic and anatomical characteristics has not hitherto proven significant [26].
Previous to these findings, pediatric neurosurgeons had also described a subtype of posterior fossa ependymomas particularly difficult to deal with [7, 16, 28]. Cerebellar-pontine angle or lateral-type posterior fossa ependymoma [28, 29, 32] refers to a type of ependymoma originating in the lateral aspect of the brainstem rather than in the floor of the fourth ventricle. They commonly present in little children as very large tumors, frequently encasing vital neurovascular structures in the posterior fossa, like the vertebral and posteroinferior cerebellar arteries, and the lower cranial nerves. As these tumors grow, they tend to displace rather than invade the surrounding tissue, and they typically displace and rotate the pons and the medulla opposite to the tumor, thus altering the anatomy and distorting the posterior fossa landmarks. Most posterior fossa ependymomas correspond to type A tumors.
Standard treatment of ependymomas consists of maximal safe surgical resection followed by focal radiotherapy [2, 3, 8, 9, 15, 18, 22, 24, 25]. Despite oncological advancements, the degree of surgical resection is still the most important prognostic factor. Surgical techniques are key to achieve successful resections, but neurological deficits after surgery are not uncommon [7, 16, 28, 29]. The management of associated hydrocephalus is also variable but may condition late follow-up. Surgical series of lateral-type posterior fossa ependymomas are seldom reported in the literature [7, 15, 16, 28, 29, 31, 32]. The clinical information they provide is invaluable, but some details are still missing (Table 1).
With this background in mind, we designed a collaborative study inside the Spanish Society of Pediatric Neurosurgery in order to evaluate the incidence of lateral posterior fossa ependymomas and the factors related to their treatment and prognosis. This information may help future surgeons face this complex disease.
Methods
This is a retrospective descriptive multicenter study. Each of the 68 active members of the Spanish Society of Pediatric Neurosurgery was contacted through the secretary of the society and invited to participate. This comprised the 30 largest hospitals in the country with a neurosurgery department treating pediatric patients, including the five pediatric neurosurgery units designed by the national health system as national centers of reference for complex pediatric neurosurgery (available at https://www.sanidad.gob.es/profesionales/CentrosDeReferencia/docs/ListaCSUR.pdf). Twenty-eight centers answered this invitation: 14 hospitals enrolled at least one patient in the study, 12 hospitals informed that they had no patients fulfilling the inclusion criteria, and two centers declined the offer due to administrative issues.
The inclusion criteria of the study were the following: a diagnosis of cerebellar-pontine angle or lateral-type posterior fossa ependymoma according to Sanford’s criteria [29] and confirmed histologically, operated in the last 10 years, with a minimum follow-up of 6 months. Preoperative and postoperative images were requested in order to confirm the correct classification of the lesion, estimate the size at diagnosis, calculate the degree of resection, and analyze the presence of hydrocephalus and other complications. Clinical data included gender, date of birth, date of surgery and at last follow-up, symptoms at presentation, and deficits present along the duration of the study. In accordance with previous investigations [29], any of the following conditions was considered a major deficit: hemiparesis, meningitis, severe cranial nerve deficit, and any potentially life-threatening circumstance. Postoperative facial nerve dysfunction was evaluated with the House-Brackmann scale [6] and considered severe when graded 3 to 5. Lower cranial nerve dysfunction was considered severe when it required transient or permanent feeding with nasogastric tube or gastrostomy and/or tracheostomy to ensure adequate ventilation and airway protection. Surgical information included the number and duration of surgeries, use of intraoperative monitoring, position of the patient, design of the incision, and use of external ventricular drain among others. Radiological data retrieved included the size and volume of the lesion at diagnosis, size and volume of residual tumor, presence of hydrocephalus at diagnosis, and ventricular size at the last follow-up according to the Evans index [5]. Degree of resection was considered complete when no residual lesion was observed in postoperative MR imaging; near-total resection (NTR) was defined as less than 1.5 cc of residual tumor on postoperative MR imaging; and subtotal resection (STR) was defined as more than 1.5 cc of residual tumors on postoperative MR images. Patients were regularly imaged to detect any tumor progression or recurrence and regularly visited in the outpatient clinic to evaluate the neurological condition. This evaluation was examined retrospectively in order to assess the quality of life in accordance to the Lansky scale [13] at 6 months and at the last follow-up.
Protocols of chemotherapy and radiotherapy treatment
Patients were treated following the International Society of Pediatric Oncology recommendations in the SIOP ependymoma study [23]. Chemotherapy was started within 3 weeks of surgery and consisted of four courses based on vincristine, etoposide, and cyclophosphamide. A second-look surgery was considered if MR after chemotherapy evidenced tumor mass. Every patient received either conventional radiotherapy or proton beam-based radiotherapy. Conventional radiotherapy consisted in a dose of 54 Gy in daily fractions of 1.8 Gy over 6 weeks. Patients older than 4 years and those younger with residual tumor received an additional boost of 5.4 Gy in three fractions to the residual tumor or to the tumor bed. When proton beam therapy was used, degraded beams of the 230 MeV cyclotron were used for treatment. For the calculation of the relative biological effectiveness, a factor of 1.1 as a relative to Cobalt 60 was used, and all doses were stated as cobalt equivalent doses.
Statistical analysis
Data from continuous variables were summarized by calculating mean values and standard deviations in descriptive analysis and evaluated with Student´s t tests when comparing independent samples. Bivariate associations were explored with chi-square and exact Fisher’s test. Survival analysis was evaluated with the Kaplan–Meier curves, and the equalities of the survival distribution for the patient groups were tested by the log-rank test. For multivariable analysis, logistic regression was used to assess the factors associated with survival at last follow-up. Level of significance was set at 0.05. SPSS version 21 (IBM Corporation, NY, USA) was used for statistical analysis.
Results
Data collection
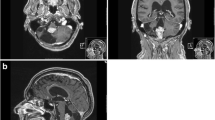
Fourteen centers included a total of 25 patients in the study. One center included 7 cases, two centers included three patients each, one center included two patients, and ten centers included one patient each. Main clinical characteristics are summarized in Table 2. There were 13 boys and 12 girls. Mean age at diagnosis was 37 months (median, 32 months). Patients most commonly complained of headache or intracranial hypertension symptoms related to associated hydrocephalus, but three patients were diagnosed due to gait disturbance and one case presented with torticollis and swallowing difficulties. The tumor extended to the left cerebellar-pontine angle in 15 patients, and in 10 patients, it extended to the right side. Mean tumor volume was over 60 cc, with three tumors measuring over 7 cm in the craniocaudal axis and extending down to C3 (Fig. 1). No case showed dissemination at diagnosis. Genetic analysis was performed in a minority of cases (2 centers, 10 patients). However, in each of these tumor specimens, immunohistochemical staining for H3K27me3 was negative, establishing the diagnosis of posterior fossa ependymoma type A.
Anatomic implications of lateral-type posterior fossa ependymoma. Upper right: axial T2 MR shows tumor extension along cerebellopontine angle, displacement and rotation of brainstem, and encasement of cranial nerves. Upper left: sagittal T1 MR depicts characteristic interrupted image of the brainstem due to tumor displacement. Tumor can extend into the cervical spinal canal both ventral and dorsal to the spinal cord. Lower right: coronal T1 MR shows elongation of middle cerebellar peduncle, encasement of PICA, and deformation of the IV ventricle. Lower left: after complete tumor resection, normal anatomic disposition is restored
Surgical procedure and main outcomes
Table 3 summarizes surgical procedures and main clinical outcomes. An external ventricular drain was inserted in 19 patients prior to resection. One of these patients developed an intratumoral hemorrhage that led to an abrupt decrease of the level of consciousness until coma, and she required an emergent posterior fossa craniectomy and partial tumor resection the night previous to the scheduled surgery. This was one of the nine patients who required two resection surgeries. Most patients were treated in a single stage, and two patients required three resection surgeries. Mean operative time was close to 10 h (median, 9.75). Gross total resection was achieved in 8 patients, near-total resection in 5 patients, and subtotal resection in 12 cases. A suboccipital approach was selected for surgery, with variable lateral extension to the affected cerebellar-pontine angle. Most patients were positioned prone, but the lateral position was selected in two cases. Different incisions were used, including midline linear, paramedian hockey stick, L-shaped, and inverted U-shaped. Intraoperative monitoring with somato-sensory and motor evoked potentials was used in every case. In four centers, free-running electromyography (EMG) of bilateral sternocleidomastoid, orbicularis oris, and masseter muscles were recorded. Genioglossus EMG and endotracheal tubes with embedded electrodes were used when necessary. Intraoperative neuronavigation was used in four hospitals, and one of them also used intraoperative ultrasonography. Although specific endoscopic-assisted surgery was not described, the micro-inspection tool QEVO (Zeiss AG, Oberkochen, Germany) was used in three cases.
Postoperative complications
In the immediate postoperative period, 14 patients developed a major morbidity: 2 required early tracheostomy and gastrostomy, 4 needed isolated tracheostomy, 6 needed mere gastrostomy, and 2 patients developed a significant hemiparesis. At 6 months, most complications had completely resolved, and only three patients showed major signs of morbidity: one requiring persistent tracheostomy, one persistent gastrostomy, and one girl showing significant hemiparesis, although she was independent to deambulate. There were two cases of mild and transient posterior fossa syndrome. A ventriculoperitoneal shunt was implanted in 18 patients due to persistent hydrocephalus, two patients were successfully treated with third ventriculostomy, and five patients developed no postoperative hydrocephalus.
One patient died in the first year after surgery: this patient showed an anaplastic ependymoma that was incompletely resected. The patient showed major deficits that required prolonged intensive care support. A significant tumor progression was detected in imaging studies, and his medical team considered that intensive support was not indicated, and the patient died 7 months after surgery. There were another five cases of exitus in the study, and 11 cases of tumor progression were documented, with a mean follow-up close to 45 months in the global series. At the median follow-up of 32 months, overall survival was 80 percent, and progression-free survival was 60%. In addition to local progression, two patients showed spinal CSF dissemination as an image finding. Thirteen patients were treated with conventional radiation therapy, and 11 received proton-based radiotherapy. At the last follow-up, the quality of life evaluated with the Lansky scale in survivors was over 90 in 16 patients, 70 in 2 patients, and 50 in one patient.
When we analyzed the factors related to postoperative major morbidity, we observed that higher histological grade was significantly associated with postoperative morbidity, whereas a higher degree of resection was not (Table 4).
Patient survival
Survival curves for overall survival and progression-free survival follow-up separated between completely and incompletely resected tumors are represented in Fig. 2. Log-rank test showed a significant association between complete resection and higher overall survival and progression-free survival. Multivariate analysis confirmed that complete resection was significantly associated with overall and progression-free survival in a binary logistic regression model with values of odds ratio over 8 (Table 5).
Discussion
Lateral-type posterior fossa ependymomas are uncommon tumors characteristic of pediatric patients [7, 15, 16, 28, 29, 31, 32], although isolated cases have been also described in adults [4, 12]. These tumors have been named after different terms, such as cerebellar-pontine angle ependymomas [28, 29], paramidline lateral posterior fossa ependymomas [15], or lateral-type posterior fossa ependymomas [7, 16, 27, 32]. This latter denomination probably reflects better the nature of these tumors and is the one that we have adopted. A unified nomenclature is necessary to correctly classify and evaluate these tumors. In particular, it is sometimes difficult to differentiate fourth ventricle ependymomas with a significant lateral extension, referred to as paramidline-medial by Ma et al. [15], from true lateral ependymomas. The surgical approach may differ in these cases: both types require a conventional approach to the fourth ventricle, but lateral-type posterior fossa ependymomas benefit from associating a direct lateral suboccipital approach and dissection along the cerebellar-pontine angle [29].
The incidence of lateral-type posterior fossa ependymomas is not well known. Relative incidence among posterior fossa ependymomas has been referred up to 40% of cases [32], although most series describe a lower incidence, around 20 to 30% of pediatric posterior fossa ependymomas [7, 15, 29]. Considering an incidence of 5.74 cases per 100,000 pediatric population of central nervous system tumors, and a relative incidence of 5% of ependymomas among them [17, 25], we could expect an incidence of 0.7 lateral-type posterior fossa ependymoma per million pediatric population. This makes lateral-type posterior fossa ependymoma a very uncommon disease. In spite of this, the incidence in our series is probably underrepresented, which may reflect both a recall bias and the absence of an adequate system of tumor registration: this indeed reveals an opportunity to improve the organization inside our pediatric neurosurgery society. Still, the present work is the second largest published series [29] and, to the best of our knowledge, the largest series in our European context.
Mean age at presentation was around 3 years, in accordance to the previous series, and distribution was balanced between both sexes. In our series, the tumor originated in the left side more frequently than in the right side, but this difference was not significant.
Most patients presented with symptoms of intracranial hypertension secondary to hydrocephalus and were treated with external ventricular drainage prior to tumor resection. Eighteen patients required a permanent ventriculoperitoneal shunt to treat persistent hydrocephalus, and 2 patients were effectively treated with a third ventriculostomy. Ventriculoperitoneal shunt may be perceived as a safer option in the short term because (1) these children are sometimes very young, and not uncommonly, they develop lower cranial nerve dysfunction after surgery; (2) neurological evaluation can be difficult and is also affected by irritability and need of intensive care treatment; and (3) there is a deadline to move to chemotherapy and radiation therapy that claims for a quick resolution of the hydrocephalus. Still, these patients show high scores in the ETVSS [10], and ventriculostomy should be considered an excellent option.
No patient showed tumor dissemination at diagnosis, but two patients were diagnosed of asymptomatic spinal seeding along follow-up. Craniospinal imaging at diagnosis and close imaging surveillance are recommended in the evaluation and follow-up of these patients. Finally, one patient developed a tumor hemorrhage soon after diagnosis. This complication has been reported exceptionally [29] but underlines the convenience of treating these patients expeditiously.
Surgical treatment of these tumors is considered a challenge. In our series, almost half of the patients required more than one resection surgery, and median surgical time was over 9 h. Gross total resection was achieved in 8 patients. Degree of total resection is greatly variable in the published series, and acquired surgical experience may be a significant factor [28, 29]. In our multicentric study, complete resections were reported from three departments, and two of them contribute with 10 cases to the series. Nevertheless, it is probable that experienced surgeons purportedly leave some tumors behind in order to preserve vital structures and achieve a maximal safe resection. However, in our study, morbidity was not higher in those patients whose resection was complete.
Major morbidity is not uncommon after lateral-type posterior fossa ependymoma surgery due to the large volume of these tumors and their dense encasement and adherence to cranial nerves and vessels of the posterior fossa. Even in most experienced hands, severe deficits develop postoperatively in almost 30% of patients [29]. In our series, 14 patients developed major deficits. However, in most cases, these deficits are resolved within 6 months. The need for a tracheostomy and a gastrostomy is a heavy burden even when transient, so families should be advised in advance and informed of the probability of recovery. Cerebellar mutism is not a characteristic complication of these tumors, but posterior fossa syndrome should be considered in the differential diagnosis of irritable infants before assuming that affective symptoms represent simple emotional reactions [21].
In our series, lateral-type posterior fossa ependymomas corresponded to posterior fossa A ependymomas in the 2021 WHO classification of central nervous system tumors in every case where H3K27me3 was studied [14]. Posterior fossa A tumors are further subdivided into PFA-1 and PFA-2 which exhibit a different gene expression [19, 20] and may have a different prognosis, whereas classic histologic grades and features of anaplasia have not shown a consistent prognostic significance. In our series, 15 cases were diagnosed as grade 2 ependymomas and 10 cases as grade 3 ependymomas. Histological grade was a non-significant factor both in bivariate and multivariate analyses when compared against the probability of tumor progression. However, a higher histological grade was significantly associated with a higher morbidity in immediate follow-up. It is hypothesized that higher grade tumors show a denser adherence and a more frequent invasion of normal parenchyma, thus making surgery more hazardous.
Postoperative focal radiation therapy forms part of the standard treatment of posterior fossa ependymoma in children. Conventional radiotherapy with photons has recently been replaced with proton therapy, which is expected to have less side effects with the same tumor control. In our series, conventional radiotherapy (54 to 59 Gy) was administered in 13 patients and proton therapy in 11 more recent cases. In one patient with grade 3 ependymoma, bad clinical condition, and early tumor progression, radiation therapy was considered not indicated, and the patient died 7 months after surgery.
Overall mortality in our series was 24% (6 exitus in 25 patients], with a mean follow-up of 44 months. Eleven patients showed tumor progression along the observation period, and 10 of these recurrences occurred by the median time of follow-up of 32 months. These results are similar to previous series, but the time of follow-up in such series is not always clearly specified. In our study, gross total resection was significantly associated with longer overall survival and progression-free survival both in bivariate and multivariate analysis.
Evaluation of quality of life is essential in the follow-up of these patients, and the use of dedicated scales like PedsQL is advisable [11], although it can be time-consuming. The play performance scale of Lansky can be used effectively as an integral part of the repeated assessment in oncologic patients and can be a first and very simple step to obtain an objective outcome in oncologic children[13]. In our series, out of 19 survivors, the latest rating in the Lansky scale was 50 in one patient, 70 in two patients, and over 90 in 16 children. More recently, a European consensus has been reached for an internationally accepted test battery for follow-up of childhood ependymoma survivors [30]. This will allow a more robust evaluation of the long-term outcomes for children and to compare these findings.
Limitations of the study
Our study has several limitations. Most importantly, this is a retrospective study with a very heterogenous participation, not only in the number of patients included by each center, but also in the diverse approach that different departments have for the same disease. Additionally, the number of cases gathered in the study underestimates the real incidence of lateral-type posterior fossa ependymomas, and the follow-up is relatively short. Probably, more recent cases—which have a shorter follow-up—have been more easily identified. This calls for the need of an operative unified national registry of pediatric central nervous system tumors, as it has been previously stated. Statistical analysis on 25 cases may be not reliable. However, all these factors do limit the power of our study but may not invalidate the significance of the associations observed.
Conclusions
Lateral-type posterior fossa ependymomas show a bad prognosis, but complete surgical resection is the first step to achieve the best results. Hydrocephalus is common, and ventriculoperitoneal shunts are frequently used, but third ventriculostomy is an ideal alternative with high success rates in most cases. Lower cranial nerve dysfunction requiring tracheostomy and gastrostomy after surgery is common, but most cases resolve within 6 months. Gross total resection should be the decided goal of surgery: it provides the best chances of overall survival and tumor control. Remarkably, gross total resection was not associated with additional morbidity. Dissemination through CSF is possible and requires a close imaging surveillance. Prospective studies are required to confirm these significant observations and guide future protocols of treatment. In this scenario, systematic evaluation of the quality of life of pediatric patients is an essential requirement.
Data Availability
Data are available through the secretary of the spanish pediatric of neurosurgery.
References
Baroni LV, Sundaresan L, Heled A, Coltin H, Pajtler KW, Lin T, Merchant TE, McLendon R, Faria C, Buntine M, White CL, Pfister SM, Gilbert MR, Armstrong TS, Bouffet E, Kumar S, Taylor MD, Aldape KD, Ellison DW, Gottardo NG, Kool M, Korshunov A, Hansford JR, Ramaswamy V (2021) Ultra high-risk PFA ependymoma is characterized by loss of chromosome 6q. Neuro Oncol 23:1360–1370. https://doi.org/10.1093/neuonc/noab034
Bertero L, Ricci AA, Tampieri C, Cassoni P, Modena P (2022) Ependymomas Pathologica 114:436–446. https://doi.org/10.32074/1591-951X-817
Delgado-López PD, Corrales-García EM, Alonso-García E, García-Leal R, González-Rodrigálvarez R, Araus-Galdós E, Martín-Alonso J (2019) Central nervous system ependymoma: clinical implications of the new molecular classification, treatment guidelines and controversial issues. Clin Transl Oncol 21:1450–1463
Dibs K, Prasad RN, Madan K, Liu K, Jiang W, Ghose J, Blakaj DM, Palmer JD, Kobalka P, Prevedello DM, Raval RR (2021) Cerebellopontine angle ependymoma presenting as isolated hearing loss in an elderly patient: a case report and literature review. Surg Neurol Int 12. https://doi.org/10.25259/SNI_781_2021
EVANS WA, (1942) An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatry 47:931. https://doi.org/10.1001/archneurpsyc.1942.02290060069004
House JW, Brackmann DE (1985) Facial nerve grading system. Otolaryngology-Head and Neck Surgery 93:146–147. https://doi.org/10.1177/019459988509300202
Ikezaki K, Matsushima T, Inoue T, Yokoyama N, Kaneko Y, Fukui M (1993) Correlation of microanatomical localization with postoperative survival in posterior fossa ependymomas. Neurosurgery 32:38–44. https://doi.org/10.1227/00006123-199301000-00006
Indelicato DJ, Ioakeim-Ioannidou M, Bradley JA, Mailhot-Vega RB, Morris CG, Tarbell NJ, Yock T, MacDonald SM (2021) Proton therapy for pediatric ependymoma: mature results from a bicentric study. Int J Radiat Oncol Biol Phys 110:815–820. https://doi.org/10.1016/j.ijrobp.2021.01.027
Jünger ST, Timmermann B, Pietsch T (2021) Pediatric ependymoma: an overview of a complex disease. Childs Nerv Syst 37:2451–2463. https://doi.org/10.1007/s00381-021-05207-7
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155:254-259.e1. https://doi.org/10.1016/j.jpeds.2009.02.048
Kulkarni AV, Piscion J, Shams I, Bouffet E (2013) Long-term quality of life in children treated for posterior fossa brain tumors. J Neurosurg Pediatr 12:235–240. https://doi.org/10.3171/2013.6.PEDS12535
Lan Z, Richard SA, Zhang Y (2019) Cerebellopontine angle ependymoma in a young adult : a case report. Medicine (United States) 98. https://doi.org/10.1097/MD.0000000000015019
Lansky LL, List MA, Lansky SB, Cohen ME, Sinks LF (1985) Toward the development of a play performance scale for children (PPSC). Cancer 56:1837–1840. https://doi.org/10.1002/1097-0142(19851001)56:7+%3c1837::aid-cncr2820561324%3e3.0.co;2-z
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, Von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Ma SC, De LC, Agazzi S, Jia W (2019) Clinical characteristics and prognostic factors of treatment in pediatric posterior cranial fossa ependymoma. Pediatr Neurosurg 54:98–107. https://doi.org/10.1159/000495809
Nagib MG, O’Fallon T (1996) Posterior fossa lateral ependymoma in childhood. Pediatr Neurosurg 24:299–305. https://doi.org/10.1159/000121059
Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS (2019) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 21:v1–v100. https://doi.org/10.1093/neuonc/noz150
Pajtler KW, Mack SC, Ramaswamy V, Smith CA, Witt H, Smith A, Hansford JR, von Hoff K, Wright KD, Hwang E, Frappaz D, Kanemura Y, Massimino M, Faure-Conter C, Modena P, Tabori U, Warren KE, Holland EC, Ichimura K, Giangaspero F, Castel D, von Deimling A, Kool M, Dirks PB, Grundy RG, Foreman NK, Gajjar A, Korshunov A, Finlay J, Gilbertson RJ, Ellison DW, Aldape KD, Merchant TE, Bouffet E, Pfister SM, Taylor MD (2017) The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 133:5–12. https://doi.org/10.1007/s00401-016-1643-0
Pajtler KW, Wen J, Sill M, Lin T, Orisme W, Tang B, Hübner JM, Ramaswamy V, Jia S, Dalton JD, Haupfear K, Rogers HA, Punchihewa C, Lee R, Easton J, Wu G, Ritzmann TA, Chapman R, Chavez L, Boop FA, Klimo P, Sabin ND, Ogg R, Mack SC, Freibaum BD, Kim HJ, Witt H, Jones DTW, Vo B, Gajjar A, Pounds S, Onar-Thomas A, Roussel MF, Zhang J, Taylor JP, Merchant TE, Grundy R, Tatevossian RG, Taylor MD, Pfister SM, Korshunov A, Kool M, Ellison DW (2018) Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol 136:211–226. https://doi.org/10.1007/s00401-018-1877-0
Pajtler KW, Witt H, Sill M, Jones DTW, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, Reimand J, Warnatz HJ, Ryzhova M, Mack S, Ramaswamy V, Capper D, Schweizer L, Sieber L, Wittmann A, Huang Z, van Sluis P, Volckmann R, Koster J, Versteeg R, Fults D, Toledano H, Avigad S, Hoffman LM, Donson AM, Foreman N, Hewer E, Zitterbart K, Gilbert M, Armstrong TS, Gupta N, Allen JC, Karajannis MA, Zagzag D, Hasselblatt M, Kulozik AE, Witt O, Collins VP, von Hoff K, Rutkowski S, Pietsch T, Bader G, Yaspo ML, von Deimling A, Lichter P, Taylor MD, Gilbertson R, Ellison DW, Aldape K, Korshunov A, Kool M, Pfister SM (2015) Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27:728–743. https://doi.org/10.1016/j.ccell.2015.04.002
Panagopoulos D, Stranjalis G, Gavra M, Boviatsis E, Korfias S, Karydakis P, Themistocleous M (2023) The entity of cerebellar mutism syndrome: a narrative review centered on the etiology, diagnostics, prevention, and therapeutic options. Children 10
Peters S, Merta J, Schmidt L, Jazmati D, Kramer PH, Blase C, Tippelt S, Fleischhack G, Stock A, Bison B, Rutkowski S, Pietsch T, Kortmann RD, Timmermann B (2022) Evaluation of dose, volume, and outcome in children with localized, intracranial ependymoma treated with proton therapy within the prospective KiProReg Study. Neuro Oncol 24:1193–1202. https://doi.org/10.1093/neuonc/noab301
Ritzmann TA, Chapman RJ, Kilday JP, Thorp N, Modena P, Dineen RA, Macarthur D, Mallucci C, Jaspan T, Pajtler KW, Giagnacovo M, Jacques TS, Paine SML, Ellison DW, Bouffet E, Grundy RG (2022) SIOP ependymoma I: final results, long-term follow-up, and molecular analysis of the trial cohort - a BIOMECA consortium study. Neuro Oncol 24:936–948. https://doi.org/10.1093/neuonc/noac012
Rudà R, Bruno F, Pellerino A, Soffietti R (2022) Ependymoma: evaluation and management updates. Curr Oncol Rep 24:985–993
Rudà R, Reifenberger G, Frappaz D, Pfister SM, Laprie A, Santarius T, Roth P, Tonn JC, Soffietti R, Weller M, Moyal ECJ (2018) EANO guidelines for the diagnosis and treatment of ependymal tumors. Neuro Oncol 20:445–456. https://doi.org/10.1093/neuonc/nox166
Sabin ND, Hwang SN, Klimo P, Chambwe N, Tatevossian RG, Patni T, Li Y, Boop FA, Anderson E, Gajjar A, Merchant TE, Ellison DW (2021) Anatomic neuroimaging characteristics of posterior fossa type A ependymoma subgroups. Am J Neuroradiol 42:2245–2250. https://doi.org/10.3174/ajnr.A7322
Sabin ND, Merchant TE, Li X, Li Y, Klimo P, Boop FA, Ellison DW, Ogg RJ (2016) Quantitative imaging analysis of posterior fossa ependymoma location in children. Childs Nerv Syst 32:1441–1447. https://doi.org/10.1007/s00381-016-3092-4
Sanford RA, Kun LE, Heideman RL, Gajjar A (1997) Cerebellar pontine angle Ependymoma in infants. Pediatr Neurosurg 27:84–91. https://doi.org/10.1159/000121232
Sanford RA, Merchant TE, Zwienenberg-Lee M, Kun LE, Boop FA (2009) Advances in surgical techniques for resection of childhood cerebellopontine angle ependymomas are key to survival. Childs Nerv Syst 25:1229–1240. https://doi.org/10.1007/s00381-009-0886-7
Thomas S, Reynolds D, Morrall MCHJ, Limond J, Chevignard M, Calaminus G, Poggi G, Bennett E, Frappaz D, Slade D, Gautier J, McQuilton P, Massimino M, Grundy R (2019) The European Society of Paediatric Oncology Ependymoma-II program Core-Plus model: development and initial implementation of a cognitive test protocol for an international brain tumour trial. Eur J Paediatr Neurol 23:560–570. https://doi.org/10.1016/j.ejpn.2019.05.009
Tomita T, Grahovac G (2015) Cerebellopontine angle tumors in infants and children. Childs Nerv Syst 31:1739–1750. https://doi.org/10.1007/s00381-015-2747-x
U-King-Im JM, Taylor MD, Raybaud C, (2010) Posterior fossa ependymomas: new radiological classification with surgical correlation. Childs Nerv Syst 26:1765–1772. https://doi.org/10.1007/s00381-010-1251-6
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Pablo Miranda, Estela Plaza and Giovanni Pancucci contributed with 7 cases to the series; Antonio López-Guerrero and Diego López-Bermeo contributed each with 3 cases to the series; Teresa García Campos and Silvia Vázquez Sufuentes contributed with 2 cases to the series; Pablo M. Munárriz, Elena López-García, Alejandra Londoño-Quiroz, Cristina Ferreras-García, Mario García-Conde, Javier Saceda-Gutiérrez, Jorge Giménez-Pando and Sara Iglesias-Moroño contributed each with one case to the series. Pablo Miranda gathered the information and wrote the manuscript. All authors reviewed and approved the text. Pablo M. Munárriz made significant corrections to the manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miranda-Lloret, P., Plaza-Ramírez, E., López-Guerrero, A. et al. Lateral-type posterior fossa ependymomas in pediatric patients: a national collaborative study. Childs Nerv Syst 40, 407–416 (2024). https://doi.org/10.1007/s00381-023-06194-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06194-7