Abstract
Fibrous hamartoma of infancy (FHI) is a rare benign soft tissue lesion of infants and young children. It usually occurs within the first 2 years of life at the superficial layer of the axilla, trunk, upper arm, and external genitalia. FHI in the central nervous system (CNS) is extremely rare. So far, only two spinal cord FHI cases have been reported. We present a case of a 1-month-old girl who presented with a skin dimple in the coccygeal area. Her MRI showed a substantial intramedullary mass in the thoracolumbar area with a sacral soft tissue mass and a track between the skin lesion to the coccygeal tip. Her normal neurological status halted immediate surgical resection. A skin lesion biopsy was first performed, revealing limited information with no malignant cells. A short-term follow-up was performed until the intramedullary mass had enlarged on the 5-month follow-up MRI. Based on the frozen biopsy result of benign to low-grade spindle cell mesenchymal tumor, subtotal resection of the mass was done, minimizing damage to the functioning neural tissue. Both the skin lesion and the intramedullary mass were diagnosed as FHI. Postoperative 5.5-year follow-up MRI revealed minimal size change of the residual mass. Despite being diagnosed with a neurogenic bladder, the patient maintained her ability to void spontaneously, managed infrequent UTIs, and continued toilet training, all while demonstrating good mobility and no motor weakness. This case is unique because the lesion resembled the secondary neurulation structures, such as the conus and the filum, along with a related congenital anomaly of the dimple.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Purpose
Fibrous hamartoma of infancy (FHI) is a rare and benign soft tissue lesion of infants and young children, most commonly occurring within the first 2 years of life. FHI is histologically characterized by a mixture of three components: mature adipose tissue, fibroblastic spindle cells, and primitive mesenchymal cells [1,2,3,4]. It was first described by Reye in 1956 as ‘subdermal fibromatous tumor of infancy’ and was named ‘fibrous hamartoma of infancy’ by Enzinger in 1965, based on the experience of 30 cases [5, 6]. According to a review of 145 cases of FHI, it was 2.7 times more prevalent in boys, and 91% occurred within the first 2 years of life. It can occur anywhere in the body and is most common in upper body parts such as the axilla (17%), upper back (16%), and upper arm (14%) [1]. Only two cases of FHI involving the central nervous system (CNS) have been reported. This is the third reported case of spinal cord FHI and the only one accompanied by a sacral subcutaneous FHI. This report aims to share the diagnostic pitfall, clinical course, and surgical treatment of this extremely rare thoracolumbar intramedullary FHI with long-term follow-up outcome.
Case presentation
A 1-month-old girl, born at 39 weeks of gestation after a normal pregnancy, presented with a sacral skin lesion. The 3 × 1.5 cm sacral dimple with edematous margin and redness was located in the midline, just above the intergluteal fold (Fig. 1). She had no family history of spinal dysraphism or neurocutaneous syndromes. The movement of her lower extremities seemed symmetric and active. The anal tone was present, and no apparent hypesthesia was detected, with intact pain sensation in her foot and ankle.
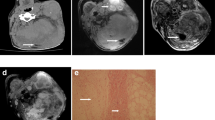
Spine sonography revealed an intramedullary mass, and MRI showed a 1.2 × 1.2 × 3.5 cm-sized thoracolumbar intramedullary expansile mass encasing the conus medullaris and extending caudally as a stalk-like structure resembling the filum terminale (Fig. 2). The skin lesion was connected to the coccygeal tip through a track-like lesion without evident intraspinal extension. Notably, the sacral subcutaneous lesion and the intramedullary mass notably exhibited similar T2 heterogeneous hyperintensity, T1 homogeneous hypointensity, and strong homogeneous gadolinium enhancement. The MRI did not clearly show a direct connection between the intradural mass and the subcutaneous lesion. Despite the unusual coexistence of a skin lesion, a malignant intramedullary tumor was suspected.
Preoperative images. a–d Initial MRI shows intramedullary mass encasing conus medullaris and filum terminale. The sacral subcutaneous lesion shows identical intensity in all sequences with intramedullary mass. The skin lesion is connected to the coccygeal tip through a track-like lesion without evident intraspinal extension (arrow) (a T2 weighted image, b T1 weighted image, c gadolinium-enhanced T1 weighted image, d fat suppression gadolinium-enhanced T1 weighted image). e Five-month follow-up MRI (gadolinium-enhanced T1 weighted image) showed interval progression of intramedullary mass and subcutaneous mass. f Preoperative C-11 methionine PET shows weak uptake at the level of T10 to T12 (arrow), cranial side of the intradural mass
Although a biopsy of the main mass seemed necessary to rule out the possibility of malignancy, her normal neurological status halted the resection of the conus lesion. As the radiological appearance of the subcutaneous lesion was almost identical to the intradural lesion, a punch biopsy of the skin lesion was performed. Skin biopsy showed dermal fibroblasts consistent with pilonidal sinus. Since evidence of malignancy was not found, we decided to perform a short-term follow-up with spine sonography. There were no interval changes in the results of spine sonographies after 1 and 2 months, and the patient remained asymptomatic. However, 4 months later, MRI showed progression of the intramedullary mass. C-11 methionine PET showed weak uptake at T10 to T12, the cranial side of the intradural mass (Fig. 2).
Preoperative electromyography (EMG) and nerve conduction study (NCS) were normal. Even though the urodynamic study (UDS) demonstrated slightly decreased bladder compliance in the middle of the filling phase, overall detrusor and sphincter activities were normal.
Surgery was performed for tissue diagnosis and decompression (Fig. 3). First, a frozen biopsy of the skin lesion was done which revealed to be benign-nature immature spindle cells with a myxoid matrix. T10 to L1 total laminectomy revealed the intramedullary mass covered with a firm white capsule. The mass looked like an enlarged conus, contiguous to the normal spinal cord. Similar to how the conus tapers to a filum, the caudal end of the bulky mass tapered to a ‘thick filum’-like structure. Notably, the ‘true’ filum terminale could not be identified. When the capsule was incised, heterogeneous soft tissue was found, including lipomatous parts with a hard consistency. A frozen biopsy of the intramedullary mass showed the same morphology as the frozen biopsy of the sacral skin lesion. The caudal resection was done at the most caudal end of the exposed operative field without extension of the laminectomy, as partial resection was inevitable in the cephalad end. Due to the absence of a clear margin between the spinal cord and the cephalad end of the mass, subtotal debulking of the main mass was performed with sufficient sparing of the neural tissue. There was no change of intraoperative motor and somatosensory evoked potentials. No motor weakness was detected postoperatively, and she was able to void spontaneously.
Intraoperative images: a T10 to L1 laminectomy is performed, and dura and spinal cord are expanded at the level of the intramedullary mass. Huge whitish, homogeneous mass is well demarcated with a smooth-surfaced capsule (arrow: functional nerve roots). b Internal debulking reveals fibrous and lipomatous tissue. c Tapered caudal end of the main mass (dotted circle: resected surface) extends in a vertical band-like structure similar to the filum. d Subtotal resection is done at a sufficient distance from the functioning cord identified through intraoperative monitoring
According to the surgical findings, the gross morphology of the suspected lesion comprised two parts. The first component represented an extraspinal portion, characterized as a soft tissue track that extended from the subcutaneous tissue in the sacral region to the distal end of the coccyx. The second component corresponded to an intradural portion, which exhibited continuity with the normal spinal cord on the cephalic side, albeit with ill-defined boundaries. On the caudal side, the lesion displayed a progressive narrowing with resemblance to the filum terminale. Importantly, the normal structures of the conus medullaris and the filum terminale could not be observed. Although the two components were close at the coccygeal tip, a direct connection was absent.
Pathologically, the patient exhibited three tumors: FHI involving the skin and subcutis, a deep fibrous histiocytoma, and an intramedullary FHI (Fig. 4). FHI shown as a dimple was histopathologically consisted of intersecting trabeculae of fibrous tissue, immature small cells arranged in the myxoid stroma, and mature fat tissue present throughout. The deep fibrous histiocytoma displayed a well-demarcated nodule composed of a prominent storiform pattern of spindle cells and osteoclast-type multinucleated giant cells. The intramedullary mass consisted of thick-walled vasculature with an immature-appearing smooth muscle media surrounding concentrically arranged immature fibrous tissue, and fat tissue, indicating FHI.
Pathology: a Fibrous hamartoma of the infancy involving skin, dermis, and subcutaneous tissue and well-demarcated nodule of deep fibrous histiocytoma is shown. b Fibrous histiocytoma of infancy shows intersecting trabeculae of fibrous tissue composed of spindle cells, immature small oval cells, and mature fat tissue presented throughout. c A nodule of deep fibrous histiocytoma displays a prominent storiform pattern of spindle cells and osteoclast-type multinucleated giant cells. d The intramedullary soft tissue hamartoma consisted of thick-walled vasculature with immature-appearing smooth muscle media and the surrounding concentrically arranged immature or myxoid fibrous tissue and fat tissue, indicating fibrous hamartoma of infancy
Postoperative MRI revealed slight expansile growth of the mass until 3 years after surgery, but there was no further growth after that for 2 years and 6 months (Fig. 5). She could walk and run well, with no motor weakness. She has been suspected of having a neurogenic bladder, although spontaneous voiding seems possible. She suffered from UTI once or twice yearly postoperatively for the first 3 years. UDS performed postoperative 5 months showed normal detrusor function, incompetent bladder neck, and bilateral vesicoureteral reflux (VUR, right side grade I, left side grade II). Postoperative 2-year follow-up UDS showed no VUR but low compliance and suspicious detrusor sphincter dyssynergia (DSD). At the last follow-up of 5.5 years, self-voiding was maintained without CIC, but toilet training had not been completed. Neurogenic bowel symptom of constipation was present, and laxatives were used routinely.
Discussion
We have presented a case of FHI consisting of an intramedullary spinal cord mass and a sacral dimple. The most significant clinical challenge in this case was the initial planning before the unexpected pathological diagnosis was confirmed. Based on the MRI findings, a malignant tumor was first suspected, but because the child had no neurological deficits, we hesitated to explore the main mass in the conus region. As the MRI features of the skin lesion on her buttock were identical to the intramedullary mass, a biopsy of the skin lesion was done first, which revealed no evidence of malignancy. Nonetheless, surgical removal was performed as enlargement of the mass size was noted. The patient was still free of neurological deficits, so partial resection was chosen to spare the functioning neural tissue. The remnant mass showed gradual and minimal growth in size until 3 years postoperatively but has been stable since then. At 5 years postoperatively, she has mild neurogenic bladder with no motor weakness.
As a significant remnant mass was present, the clinical issue was whether adjuvant treatment of the remnant mass should be considered. The classic FHI found as a subcutaneous mass generally showed a good prognosis after surgical resection. Even with incomplete resection, less than 15% of cases showed recurrence [1, 7]. No metastasis has been reported. Local recurrence is still curable with reoperation, and recurrence after reoperation is extremely rare [1, 4, 8]. Another report stated that untreated FHI grows rapidly during the first 5 years of life, and then it slows down [2]. The long-term outcome of the present case aligns with the general trend observed in FHI. It demonstrated subtle, expansile growth until the age of 3 and then stabilized.
In 1996, Tortori-Donati et al. reported a case of the spinal cord and subcutaneous mass, consisting of various components, including FHI, in a 2-year-old child with delayed motor development and thoracolumbar subcutaneous mass. The MRI examination revealed a subcutaneous mass at the T9-10 level, which lacked any connection to the intraspinal region. Additionally, they discovered a contrast-enhanced intradural mass that enveloped the spinal cord at the T10 to L3 level. Histologically, the superficial layer of the subcutaneous mass displayed features of FHI, while the deeper layer mainly exhibited characteristics of giant cell angioblastoma. The intradural mass demonstrated distinctive features corresponding to the ‘diffuse–type’ infantile fibromatosis [9]. The authors described the spinal cord and subcutaneous mass as ‘fibroblastic proliferation.’ However, the report did not provide specific details regarding whether FHI was solely limited to the subcutaneous mass or if it also constituted a portion of the intradural lesion. Moreover, they focused primarily on the peculiar diagnosis without demonstrating a postoperative course following surgical resection.
The second case was about a 10-month-old boy presented with right-side dominant lower extremity weakness. His initial MRI revealed extensive intramedullary mass from T10 to L4 with intratumoral hematoma at the L2 level. Extensive surgical excision was planned. However, since cauda equina roots were compressed to the right side and attached tightly to the mass, minimal resection was sufficient for tissue diagnosis. Pathologic finding was reported as FHI with interspersing glial cells. They reported an 8-month follow-up after surgery, and the remnant tumor had no further growth. The child showed no change in his neurological status [7].
The histogenesis of FHI has generally garnered little interest as it involves mesenchymal origin cells in the subcutaneous region. As the present case differed from subcutaneous FHI in its atypical location and morphology, we expected its pathogenesis to be related to an error during neural tube formation. The present case involved the lower conus and structure resembling the filum with a sacral skin lesion, which is all known to be formed by secondary neurulation. The main player of secondary neurulation is the caudal cell mass (CCM), an undifferentiated cell mass of mesenchymal origin [10]. The speculation arises that the loose immature mesenchyme and its derivatives, such as adipose tissue and intersecting trabeculae of fibrous tissue, may have formed from the CCM. The most common congenital anomaly related to secondary neurulation is spinal dysraphism, including lumbosacral lipoma and thickened fatty filum. It is well known that complex types of lumbosacral lipomas contain not only adipose tissue but also neural tissue, muscle, fibrous cells, and even bone [11]. Nonetheless, it is intriguing that although the cellular component is ‘abnormal,’ the ‘normal’ regression phase of the secondary neurulation has been at least partially completed. However, the coexistence of skin lesions in this case, and the occurrence of upper spinal cord lesions in other reported cases, cannot be solely explained by the differentiation of CCM. Due to the scarcity of relevant precedents, the true pathogenesis remains questionable.
Conclusions
We reported a case of intramedullary and coexisting subcutaneous FHI presented as a sacral dimple with a postoperative 5-year follow-up. Our case is the third reported spinal cord FHI with the longest follow-up to 5 years. Also, our case is the only case accompanied by a subcutaneous lesion in the sacral area and the caudal extension of the intramedullary mass to a band resembling the filum. Considering the nature of the disease, maximal resection need not be attempted for pathologically confirmed FHI. Further long-term follow-up of the case will be needed.
References
Al-Ibraheemi A, Martinez A, Weiss SW, Kozakewich HP, Perez-Atayde AR, Tran H, Parham DM, Sukov WR, Fritchie KJ, Folpe AL (2017) Fibrous hamartoma of infancy: a clinicopathologic study of 145 cases, including 2 with sarcomatous features. Mod Pathol 30:474–485
Efem SE, Ekpo MD (1993) Clinicopathological features of untreated fibrous hamartoma of infancy. J Clin Pathol 46:522–524
Ji Y, Hu P, Zhang C, Yan Q, Cheng H, Han M, Huang Z, Wang X, Li H, Han Y (2019) Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord 20:356
Saab ST, McClain CM, Coffin CM (2014) Fibrous hamartoma of infancy: a clinicopathologic analysis of 60 cases. Am J Surg Pathol 38:394–401
Reye RD (1956) A consideration of certain subdermal fibromatous tumours of infancy. J Pathol Bacteriol 72:149–154
Enzinger FM (1965) Fibrous hamartoma of infancy. Cancer 18:241–248
Yano S, Hida K, Nagashima K, Iwasaki Y (2004) Spinal fibrous hamartoma of infancy: case report. Neurosurgery 55:712
Miyamoto M, Tsunoda R, Gembun Y, Konno S, Hagiwara Y, Liu X, Ito H (2010) Recurrence of fibrous hamartoma of infancy excised 14 years after the primary surgery. J Neurosurg Pediatr 5:136–139
Tortori-Donati P, Fondelli MP, Rossi A, Andreussi L, Brisigotti M, Garre ML (1996) MRI in an unusual case of congenital spinal mesenchymal proliferation. Neuroradiology 38(Suppl 1):S196-199
Yang J, Lee JY, Kim KH, Wang KC (2021) Disorders of secondary neurulation: mainly focused on pathoembryogenesis. J Korean Neurosurg Soc 64:386–405
Sim J, Shim Y, Kim KH, Kim SK, Lee JY (2021) Features of the filum terminale in tethered cord syndrome with focus on pathology. J Korean Neurosurg Soc 64:585–591
Funding
This work was supported by the National Research Foundation (NRF) funded by the Korean Government, the Ministry of Science and ICT (MSIT) (2018R1A5A2025964).
Author information
Authors and Affiliations
Contributions
TH Park reviewed the case and conducted a literature search, then wrote the first draft, and composed the final manuscript based on revisions from other authors. KH Kim, SK Kim, KC Wang contributed to significant revisions of the draft, read and agreed the final manuscript. SH Park reviewed the pathological findings of the case, contributed to the draft revision, particularly the parts related to pathology, and read and agreed to the final manuscript. JY Lee was the primary consultant for this patient, provided ideas for the initial concept of the manuscript, wrote and revised the manuscript, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, TH., Kim, K.H., Kim, SK. et al. Fibrous hamartoma of infancy of the spinal cord resembling conus and filum, with a coexisting sacral dimple. Childs Nerv Syst 40, 245–251 (2024). https://doi.org/10.1007/s00381-023-06133-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-023-06133-6