Abstract
Non-terminal myelocystoceles are commonly found in the cervical or thoracic spinal region. Their sac can rarely be associated with tumor. A rare case of an infant with a lumbosacral non-terminal myelocystocele and accompanying mature teratoma is reported in whom the tumor was attached to the placode not as a part of the sac.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-terminal myelocystocele exhibits herniation of the posterior wall of the spinal cord through a defect in the dura and posterior spinal canal [1,2,3,4]. Very few non-terminal myelocystoceles are located in the lumbosacral spinal cord [1, 5]. They are usually covered with a skin layer [5, 6] and, in most instances, are associated with normal neurological development [6,7,8,9,10]. In all cases, sac resection with adequate untethering of spinal cord and dural reconstruction remains the mainstay of standard treatment [11, 12].
The coexistence of tumors and myelocystoceles are extremely rare [13]. Despite the fact that spinal dysraphism is adequately discussed in the literature, very few reports are published in this respect. Among those reported, teratoma [4] and dermoid/epidermoid tumors [14, 15] were most described. We describe a rare case of a non-terminal myelocystocele located in the lumbosacral region and associated with a teratoma attached to the neural placode. The clinical presentation and surgical treatment are discussed.
Case report
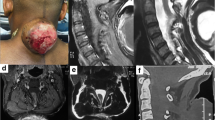
A 6-month-old male infant was admitted with a preliminary diagnosis of a lumbosacral mass. He was a normal healthy infant with a head circumference on the 75th percentile according to his age. On physical examination, a 6 × 8 × 10 cm posterior midline swelling was observed in the lumbosacral region which was covered with poor quality and dysplastic skin (Fig. 1A). His neurological exam was normal. No hydrocephalus or CSF leak was noticed.
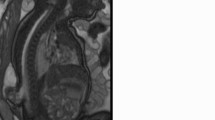
Spinal MRI imaging revealed a fluid-filled cyst, non-homogenously hypointense on T1 and hyperintense on T2-weighted images. The cord was extended outside the spinal defect with an expanded trumpet-like disfiguration, and attached to a cystic solid non-homogenous mass at the suspicious placode site (Fig. 1B). A urodynamic study revealed a neurogenic bladder with high pressure bladder and detrusor sphincter dyssynergia.
During operation a cystic solid 2–3-cm-diameter mass was found inside the expanded placode extending anteriorly to the center of neural placode and central canal, and posteriorly into the myelocystocele. Surgery was performed with cord untethering, gross total resection of mass by meticulous dissection of the tumor from neural placode (which had a good dissection plane), sac resection, pial suturing, and duraplasty associated with fascia and skin closure in the layers. Distal end of cord and cauda equine were inside the spinal canal distal to myelocystocele.
Histopathological finding of the tumor revealed a mature cystic teratoma consisting of endodermal component (respiratory and intestinal epithelium and salivary glands), mesodermal component (fibro-fatty tissue, mature cartilage, and nerve) and ectodermal component (skin adnexae and hair follicles) (Fig. 2A, B).
A. Mature teratoma with ectodermal components, consists of cyst wall lined by squamous epithelium with associated hair follicles and sebaceous glands. (H&E × 10). B. Section of tumor with mesodermal (fibro-fatty tissue and hyaline cartilage) and endodermal (respiratory epithelium and salivary glands) components. (H&E × 10)
Postoperative course was unremarkable. His 1-year follow-up is uneventful with normal developmental status during regular neurosurgical visits without recurrence in repeated MRI.
Discussion
Spinal dysraphisms are categorized as open or closed anomalies [5, 16]. Based on their clinico-pathological findings open spinal dysraphisms may be grouped as myelomeningocele, myelocele (myeloschisis), hemimyelomeningocele and hemimyelocele, whereas myelocystoceles; lipomyelomeningocele, and lipomyelocele form closed occult spinal dysraphisms [5, 17,18,19,20]. Lipomyelomeningoceles are the most common forms of occult spinal dysraphisms [21]. Myelocystoceles are characterized as terminal or non-terminal [4]. A non-terminal myelocystocele is a large skin-covered CSF-filled meningocele that may be attached to the spinal cord or lie continuous with the central canal of the hydromyelic spinal cord [5]. On the 3rd to the 4th week of gestation, primary neurulation occurs under normal embryological parameters. Any disruption that leads to the incomplete fusion of the neural fold during primary neurulation results into dilation of the central canal of the caudal spinal cord with a pia matter lining filled with CSF (syringocele) that is surrounded by an expanded dural sheath with arachinoid lining (meningocele) [22, 23]. There are two main factors responsible for this defect. First, no expression of bone morphogenic protein (BMP) leads to impaired fusion of the ectoderm and abnormal connection between the ectoderm and neuroectoderm. Second, focal myeloschisis is due to incomplete fusion of the neural fold [22].
Most non-terminal myelocystoceles are located in the cervical or thoracic [8], mostly covered with full-thickness skin and often exhibit no neurological dysfunction [1, 4]. Lumbosacral non-terminal myelocystoceles are extremely rare, and very few cases are reported in literature. [1, 5, 13].Teratomas are the most frequent tumors associated with dysplactic cystic lesions of spine or other kinds of spinal dysraphisms [4], which may occur as an isolated tumor or embedded in dysraphic anomalies. Different reports of such association have been found including teratoma-associated myelomeningocele or myelocystocele [4, 13], tumor with thick filum terminale [24], a teratoma originated from and embedded in filum terminale [25] or even teratoma mimicking a meningomyelocele [4, 5].
Development of teratoma was proposed to be a dysembryonic mechanism which the tumor originates from pluripotent cells of caudal cell mass through various types of cell-signaling dysfunction [26]. Although sacrococcygeal teratomas are commonly diagnosed prenatally via ultrasound, teratomas associated with spinal dysraphisms may not be evident on clinical exam and preoperative imaging. Therefore the most reliable means of diagnosis is via good intraoperative observation and histopathological examination [4, 13].
A few reports of association of myelocystocele and teratoma have been found (1, 22). All of them were dysraphic lesions in which the tumor was embedded inside the sac, and pathological examination confirmed the accompanying tumor (1, 22, 23). There is no published case with tumor totally attached to neural placode that has surgical importance per se. If the tumor is attached to the neural placode as in this case, meticulous dissection of tumor from neural placode specially in patients with normal motor exam is warranted to prevent any subsequent neurological deficit. On the other hand, gross total resection of tumor is mandatory to prevent from any remnant of embryonic tumor which can be the source of tumor growth and subsequent surgeries in the future. Therefore, close follow-up with neuroimaging is necessary to avoid probable recurrence.
Conclusion
Association of tumor and myelocystocele is very rare which mostly the tumor is a part of the sac. Here we reported a rare case of lumbosacral non-terminal myelocystocele which a soft tissue mass (teratoma) was attached to the placode and was resected with meticulous dissection. We believe this is the first case of a lumbosacral non-terminal myelocystocele associated with a teratoma located on the placode.
References
Gressot LV, Mohila CA, Jea A, Luerssen TG, Bollo RJ (2014) Cervicothoracic nonterminal myelocystocele with mature teratoma: case report. J Neurosur Pediatr 13(2):204–208. https://doi.org/10.3171/2013.12.PEDS13408
Rossi A, Piatelli G, Gandolfo C, Pavanello M, Hoffmann C, Van Goethem JW, Tortori-Donati P (2006) Spectrum of nonterminal myelocystoceles. Neurosurgery 58(3):509–515. https://doi.org/10.1227/01.NEU.0000197122.92954.82
Muthukumar N (2007) Terminal and nonterminal myelocystoceles. J Neurosurg Pediatr 107(2):87–97. https://doi.org/10.3171/PED-07/08/087
Feltes CH, Fountas KN, Dimopoulos VG, Escurra AI, Boev A, Kapsalaki EZ, Troup EC (2004) Cervical meningocele in association with spinal abnormalities. Childs Nerv Syst 20(5):357–361
Delashaw JB, Park TS, Cail WM, Vollmer DG (1987) Cervical meningocele and associated spinal anomalies. Childs Nerv Syst 3(3):165–169. https://doi.org/10.1007/BF00717894
Matson DD (1969) Neurosurgery of infancy and childhood. Thomas
Şanlı AM, Kertmen H, Karavelioglu E, Şekerci Z (2008) Giant true dorsal thoracic meningocele in a school-age child: case report. J Neurosurg Pediatr 1(5):399–401. https://doi.org/10.3171/PED/2008/1/5/399
Steinbok P, Cochrane DD (1991) The nature of congenital posterior cervical or cervicothoracic midline cutaneous mass lesions: report of eight cases. J Neurosurg 75(2):206–212. https://doi.org/10.3171/jns.1991.75.2.0206
Flanders TM, Franco AJ, Hines SJ, Taylor JA, Heuer GG (2020) Neonatal intraoperative neuromonitoring in thoracic myelocystocele: a case report. Childs Nerv Syst 36(2):435–439. https://doi.org/10.1007/s00381-019-04380-0
Sakai K, Sakamoto K, Kobayashi N, Iguchi H (1996) Dermoid cyst within an upper thoracic meningocele. Surg Neurol 45(3):287–291. https://doi.org/10.1016/0090-3019(95)00245-6
Scott RM, Wolpert SM, Bartoshesky LE, Zimbler S, Klauber GT (1986) Dermoid tumors occurring at the site of previous myelomeningocele repair. J Neurosurg 65(6):779–783. https://doi.org/10.3171/jns.1986.65.6.0779
Erol FS, Topsakal C, Ozveren MF, Akdemir I, Cobanoglu B (2004) Meningocele with cervical dermoid sinus tract presenting with congenital mirror movement and recurrent meningitis. Yonsei Med J 45(3):568–572
Venkataramana (2011) N.J.J.o.p.n Spinal dysraphism 6(Suppl1):S31
French BN (1983) The embryology of spinal dysraphism. Clin Neurosurg 30:295–340. https://doi.org/10.1093/neurosurgery/30.cn_suppl_1.295
Kim YH, Jahng TA, Chung CK (2006) Coexistence of subcutaneous dermoid cyst and lipomyelomeningocele. Journal of Korean Neurosurgical Society 39(4):310–313
Abu-Bonsrah N, Purvis TE, Goodwin CR, Petteys RJ, De la Garza-Ramos R, Sciubba DM (2016) Adult cervicothoracic lipomyelomeningocele. J Clin Neurosci 32:157–159. https://doi.org/10.1016/j.jocn.2016.04.005
Pang D, Dias MS (1993) Cervical myelomeningoceles. Neurosurgery 33(3):363–373. https://doi.org/10.1097/00006123-199309000-00003
Andronikou S, Wieselthaler N, Fieggen AG (2006) Cervical spina bifida cystica: MRI differentiation of the subtypes in children. Childs Nerv Syst 22(4):379–384
Yu JA, Sohaey R, Kennedy AM, Selden NR (2007) Terminal myelocystocele and sacrococcygeal teratoma: a comparison of fetal ultrasound presentation and perinatal risk. Am J Neuroradiol 28(6):1058–1060. https://doi.org/10.3174/ajnr.A0502
Sugitani M, Morokuma S, Hidaka N, Kinoshita Y, Taguchi T, Tsukimori K, Wake N (2009) Three-dimensional power Doppler sonography in the diagnosis of a cystic sacrococcygeal teratoma mimicking a meningomyelocele: a case report. J Clin Ultrasound 37(7):410–413. https://doi.org/10.1002/jcu.20592
Sherer DM, Fromberg RA, Rindfusz DW, Harris BH, Sanz LE (1997) Color Doppler aided prenatal diagnosis of a type 1 cystic sacrococcygeal teratoma simulating a meningomyelocele. Am J Perinatol 14(01):13–15
Albert G, Campos M, Menezes A, Vogel T, Weinstein S (2008) Rachipagus parasite associated with myelocystocele and diastematomyelia. Pediatr Neurosurg 44(5):418–421. https://doi.org/10.1159/000149912
Maiti TK, Bhat DI, Devi BI, Sampath S, Mahadevan A, Shankar SK (2010) Teratoma in split cord malformation: an unusual association: a report of two cases with a review of the literature. Pediatr Neurosurg 46(3):238–241. https://doi.org/10.1159/000320386
Fernández-Cornejo VJ, Martínez-Pérez M, Polo-García LA, Martínez-Lage JF, Poza M (2004) Neurocirugia (Astur) 15(3):290–293
Ates O, Cayli SR, Koçak A, Alkan A, Onal C, Usta U (2005) Neurol Med Chir (Tokyo) 45(7):375–378
Oliveria SF, Thompson EM, Selden NR (2010) Lumbar lipomyelomeningocele and sacrococcygeal teratoma in siblings: support for an alternative theory of spinal teratoma formation. J Neurosurg Pediatr 5(6):626–629
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors of this paper do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berchi Kankam, S., Ashrafi, M., Tayebi Meybodi, K. et al. Lumbosacral non-terminal myelocystocele associated with teratoma: case report and review of literature. Childs Nerv Syst 38, 1229–1232 (2022). https://doi.org/10.1007/s00381-021-05361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05361-y