Abstract
Objective
This study documents the monitorability using different anesthesia regimes and accuracy of muscle motor evoked potentials (mMEPs) in children ≤2 years of age undergoing tethered cord surgery (TCS).
Methods
Intraoperative mMEP monitoring was attempted in 100 consecutive children, ≤2 years of age, undergoing TCS. MEP monitoring was done under 4 different anesthetic regimes: (Total intravenous anesthesia (TIVA); balanced anesthesia with sevoflurane and ketamine; balanced anesthesia with isoflurane and ketamine; and balanced anesthesia with sevoflurane). Factors analyzed for their effect on monitorability were: age, neurological deficits, type of anesthesia, and the number of pulses used for stimulation.
Results
Baseline mMEPs were obtained in 87% children. Monitorability of mMEPs was similar in children ≤1 year and 1-2 years of age (85.7% and 87.5%). In multivariate analysis, anesthesia regime was the only significant factor predicting presence of baseline mMEPs. Children undergoing TIVA (p=0.02) or balanced anesthesia with a combination of propofol, sevoflurane, and ketamine (p=0.05) were most likely to have baseline mMEPs. mMEPs had a sensitivity of 97.4%, specificity of 96.4%, negative predictive value of 98.2% and accuracy of 96.8%.
Conclusions
Baseline mMEPs were obtained in >85% of children ≤2 years of age including those who had motor deficits. TIVA and balanced anesthesia with sevoflurane and ketamine are ideal for mMEP monitoring. mMEPs have a high accuracy although, false positive and false negative results can occasionally be experienced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tethered cord surgery (TCS) is often performed in children <2 years of age. In conditions such as lipomyelomeningocele (LMMC), terminal myelocystocele and split cord malformation type 1 (SCM 1), the corticospinal tract is at risk during surgery. Thus, multi-modality intra-operative neurophysiological monitoring (IONM) with muscle motor evoked potentials (mMEP), free running electromyography (EMG) and triggered EMG are required for such surgeries.
For several years, incomplete myelination of the corticospinal tract was cited as a reason for not using transcranial motor evoked potentials (TcMEPs) in very young children (<2 years of age) [1, 2]. Need for high voltages of transcranial electrical stimulation to obtain mMEP responses in young children has also led to concerns about safety [3].
In 2011, Fulkerson et al. [4], published the first report on the successful use of intra-operative mMEP monitoring in 10 children <3 years of age undergoing spinal surgeries. However, the utility of IONM using mMEPs during TCS has not been studied in a large cohort of children ≤2 years of age.
We studied the safety, feasibility and the diagnostic accuracy of mMEPs in a large cohort of children, ≤2 years of age undergoing TCS. We also studied the effect of different anesthetic regimes on intra-operative mMEP monitoring.
Methods
Patients
This retrospective study included 100 consecutive children ≤2 years of age undergoing TCS with multi-modality IONM, from August 2014 to September 2019.
The study was approved by the institutional review board (IRB No. 13095; dated 24.06.2020).
Anesthesia regimes
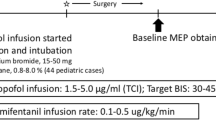
Anesthesia was induced using sevoflurane and deepened with bolus dose of fentanyl 2 mcg/kg and propofol 1–2 mg/kg. Paralysis was attained with atracurium 0.5 mg/kg. For the maintenance of anesthesia, four protocols were followed (Table 1). The depth of anesthesia was monitored keeping the Bispectral Index (BIS) between 40 and 60. The blood pressure was maintained within 15% of the baseline. After ensuring all four twitches in ‘Train of Four’ neuromuscular monitor, baseline mMEPs were recorded before the skin incision.
mMEP technique
Multipulse transcranial electrical stimulation [Cadwell, Endeavour (Nicolet Biomedical Inc), USA] was delivered using a train of 5 or 7 pulses (pulse width 75 μs) at 300 Hz. Stimulus was delivered through two cork screw electrodes placed at C1, C2 (10/20 International electroencephalography system) region. Subdermal needle electrodes were used to record mMEPs bilaterally from quadriceps, tibialis anterior (TA), soleus, extensor digitorum brevis (EDB), abductor hallucis (AH) and external anal sphincter (EAS) muscles. Stimulation was started at a voltage of 60 V and gradually increased in steps of 10 volts until responses could be obtained from all the muscles. The maximum voltage used before concluding absent baseline responses was 320 V, checked initially at 5 pulses and then at 7 pulses. A single train of stimuli was used and responses were recorded for a duration of 100 ms. Band pass filters were set between 10 Hz and 500 Hz.
Recordings were repeated at least twice till the completion of laminectomy. After the dura was opened, intermittent recordings were performed on an average of every 10 to15 min or more frequently at critical steps of the surgery. Typically, recordings were obtained after excision of the lipoma, completion of the untethering and neurulation of the placode in children with a lipoma. A final recording was obtained after dural closure.
In addition to mMEPs, our routine multi-modality IONM for TCS includes free running EMG, triggered EMG and the bulbo-cavernosus reflex.
Surgical technique
All children with lipomas were operated with the intent of safe, radical excision guided by mMEP monitoring [5]. Any associated tethering elements like fatty filum were also sectioned. During resection of the bony spur in SCM, medial dural cuff was always removed along with sectioning of the medial non-functional roots.
Outcome variables
Monitorability was determined on the basis of the ability to record baseline mMEP responses in any one of the lower limb muscles. mMEP responses were deemed “adequate” when stimulation yielded reproducible waveforms of sufficient duration and complexity, with amplitudes of at least 50 μV in at least one monitored muscle group.
Improvement in mMEPs was defined as an increase in amplitude of the mMEPs of >50% or ability to record mMEPs in muscles from which baseline responses were not obtained. Alarm criteria were set for a decrease in the amplitude of mMEPs by >50% in any one muscle. This was also noted as worsening of mMEPs.
Motor improvement in the post-operative period was defined as improvement in motor power (if there was pre-operative dysfunction) in any one of the monitored muscles by 1 grade or more of the Medical Research Council (MRC) grading system. Similarly, motor worsening was defined as a decrease in motor power in any one of the monitored muscles by 1 grade or more.
Bladder dysfunction was defined as the presence of urinary urgency, incontinence or increased urinary frequency in older children or absence of periods of dry pads in infants. Bladder dysfunction was further evaluated with ultrasound abdomen, cystourethrogram, uroflometry and DMSA (dimercapto succinic acid) scan.
Diagnostic accuracy
To calculate the sensitivity and specificity, we used the following definitions:
Positive test: Any significant change (improvement or worsening of mMEPs) at the end of surgery;
Negative test: mMEPs remaining the same as at the baseline at the end of surgery;
True positive: mMEPs improved and motor power/bladder function remained the same or improved after surgery; or mMEPs worsened with deterioration of motor power/bladder function after surgery;
False positive: MEPs improved but power deteriorated; or MEPs worsened but motor power/bladder function remained same or improved.
True negative: MEPs remained the same and motor power/bladder function also remained the same or improved;
False negative: MEPs remained the same but motor power/bladder function worsened.
Statistical analysis
All categorical variables were reported using frequencies and percentages and continuous variables were expressed in terms of mean and standard deviation. Penalized logistic regression was done to find the association of risk factors with MEP response. Statistical significance was measured at p<0.05. All the statistical analysis was performed using Stata software version 16.0 (Stata corp, USA). Standard formulae were used to calculate sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios and accuracy.
Results
Patient demographics and pathology
Mean age of the children was 17 ± 5.8 months (range 3–24 months). Neurological deficits in the lower limbs were present in 50 children, of whom 20 had severe weakness (MRC grade 0–2). Bladder dysfunction was seen in 49 children. Lipomyelomeningocele was the commonest diagnosis, noted in 72 (72%) children followed by SCM and dermal sinus in 12 (12%) children each.
Effect of anesthesia regime (Table 1)
The highest success rate for recording baseline mMEPs was with TIVA and balanced anesthesia with ketamine (92.8 and 90.9%, respectively). Success rates were lower when isoflurane or a regimen without ketamine was used.
Stimulation voltage
The stimulation voltage used to elicit baseline mMEPs ranged from 180 V to 320 V (mean, 255.9 ± 36 V). In children ≤1 year it ranged from 190 V to 300 V (mean, 254.6 ± 35.7 V) and 180 V to 320 V (mean, 256.4 ± 36.4 V) in those 1 to 2 years of age.
Monitorability (Table 2)
Overall, baseline mMEPs were obtained in 87% of children. Baseline recordings were obtained in 24/28 (85.7%) of children ≤1 year of age and 63/72 (87.5%) of children 1 to 2 years of age. Baseline mMEP responses were obtained from 47 (94%) children with motor deficits while it was obtained in 80% children with no deficits (p = 0.05). Similarly, a higher proportion of children undergoing TIVA had baseline mMEP responses than those who received inhalational anesthetics (p = 0.05).
In the multivariate analysis, anesthesia regime was found to be the only significant factor associated with the probability of obtaining baseline mMEPs. Anesthesia regime with TIVA was associated with the highest chances of obtaining a mMEP response (p = 0.02). Among the combined regimens, a combination of propofol, sevoflurane and ketamine (p = 0.04) had the highest rate of baseline responses. Preoperative motor deficits did not retain significance in the multivariate analysis indicating that it was the differences in the anesthesia regime rather than motor deficits which was responsible for the higher baseline mMEP rates in children with motor deficits.
Among the 13 children with no baseline mMEPs, responses were obtained in seven after the un-tethering, while six children had no mMEP responses throughout the surgery. In the six children with no responses throughout surgery, five (all with LMMC) had no preoperative deficits while another child with SCM 1 had only grade 3/5 power of the lower limbs.
Changes in mMEPs
An improvement in mMEPs (positive test) was noted in 36% children. In 7 children, mMEPs were obtained from muscles which had no baseline mMEPs and in 29 children the amplitude of the baseline mMEPs increased following the un-tethering (Figs. 1 and 2).
Sagittal T1W (a) and T2W (b) MR images showing a L3-5 intradural lipoma extending to the subcutaneous plane along with a thickened fatty filum in an 11-month-old girl who presented with the history of a swelling in the lower back since birth without any limb weakness or bladder dysfunction. (c) There were no baseline mMEPs. MEP responses were obtained from left soleus after L2 to S1 laminoplasty, near total excision of transitional lipoma and sectioning of thickened filum
Sagittal (a) and axial (b) T1W MR images of a 2-year-old boy with low back swelling and urinary incontinence showing L4-5 lipoma in continuity with a subcutaneous lipoma. Baseline (c) and post-untethering (d) MEP recordings show improved amplitude of MEPs from left EDB and AH and bilateral TA (arrows), after L2 to L5 laminoplasty, near total excision of transitional lipoma and untethering of cord
mMEPs worsened (positive test) in 3 children during surgery though there was no clinical worsening in two of them (Table 3).
In the 55 children, there was no change in the mMEPs at the end of surgery.
Complications of mMEP monitoring
There were no seizures, tongue bites, skin burns at stimulation sites or cardiac arrhythmias observed in any child.
Early clinical outcomes
Most of the children (93%) remained clinically the same after the surgery while motor power improved in 4 (4%) children (Table 3). There was deterioration of the muscle power in the lower limbs in 3 children (one child had no baseline mMEPs, mMEPs amplitude reduced by 60% in the second one and mMEPs remained the same in the third one.) No child with a normal bladder function developed a postoperative urinary dysfunction; 25/49 of children with preoperative bladder dysfunction underwent a perioperative bladder catheterization.
False positive and false negative cases
A true negative test was obtained in 54 children and a false negative in one child. The false negative result was in an 18-month-old child with a lumbosacral LMMC and no deficits. She developed weakness of the left ankle (grade 2 power) after surgery though mMEPs from bilateral TA, soleus, EDB and AH remained the same throughout the surgery (Fig. 3). The ankle power started improving within a week after surgery and she had normal power at one year follow-up.
Sagittal (a) and axial (b) T1W MR images showing a lipomyelomeningocele with asymmetric type of transitional lipoma in an 18-month-old child with low back swelling without any neurological deficits. The baseline mMEPs (c) were unchanged at the end of surgery (d). However, the child developed weakness of left ankle with grade 2/5 power which improved to grade 3/5 a week after surgery and normal power (5/5) at one year follow-up
A true positive test was noted in 37 children. In 36 children, mMEPs improved and motor power remained the same (34 children) or improved (2 children). In one child motor weakness was associated with a drop in mMEPs (Fig. 4). In two children, mMEPs loss was not associated with a neurologic deficit (false positive).
(a) Sagittal T2W MR image of an 11-month-old child with swelling in the mid back since birth and progressive weakness of the left hand showing a nonterminal myelocystocele in the lower thoracic and upper lumbar region and a syrinx extending into the cervical cord. (b) Axial T2W MR image showing the myelocystocele. (c) Baseline mMEPs were obtained from all the lower limb muscles bilaterally. (d) There was >50% reduction in the amplitude of mMEPs from right TA and quadriceps (arrows) after repair of the myelocystocele. Post-operatively there was a weakness of right lower limb muscles to grade 2/5 which improved to grade 3/5 at one week after surgery
Thus, the false positive and false negative rates of mMEPs to detect postoperative changes in motor function were 3.6 and 2.6%, respectively (Table 4).
Diagnostic accuracy of mMEPs
The diagnostic statistics of mMEPs are shown in Table 4. mMEPs had a high a sensitivity (97.4%) and specificity (96.4%) in predicting motor changes during surgery. Diagnostic accuracy was 96.8%.
Discussion
Effect of age on mMEPs
Several authors have commented that mMEPs might be difficult to obtain in young children especially those <6 years of age [6] and almost impossible in children <2 years of age [1] due to immaturity of the cortico-spinal tracts with incomplete myelination and synaptogenesis [3]. mMEP recordings are better obtained in the upper limbs due to larger cortical representation of the hand muscles and therefore, a larger volley of current travelling through the cortex to the muscles [1, 6,7,8].
Baseline mMEPs can be obtained in nearly 80% of children <6 years of age although, there is a progressive increase in monitorability with increasing age of the child. However, the stimulating voltage required to obtain baseline mMEPs in children has been shown to be considerably higher than that required in adults [3]. Aydinlar et al. [1] used a mean threshold of 488.46 ± 99.76 V (range 310–740 V) for TcMEPs in infants undergoing spinal cord surgeries. On an average, voltage of >300 V is generally required to obtain recordings from the lower limb muscles. Fulkerson et al. [4] could record baseline mMEPs in all the 10 children (age <3 years) studied. However, the mean baseline stimulating voltage was much higher in their study; 533 ± 124 V (range 321–746 V), compared to 255.9 ± 36 V (range180–320V) in our study. Similarly, Yi et al. [9] used a stimulating voltage as high as 900V (mean 596 ± 154 V), for eliciting MEPs in infants <3 months undergoing surgery for spinal and cranial pathologies. They could obtain mMEP responses from 24/25 children in at least one limb. In our study, the mean voltage was slightly lower at around 285 V. Although, adverse effects of high stimulation voltage on the brain of young children are not mentioned in the literature, we feel that it would be prudent to use the lowest possible voltages needed to obtain baseline mMEPs.
In contrast to the foregoing studies, Senkoylu et al. [2] could obtain MEP responses in all children below 11 years of age undergoing spinal surgery with a low stimulating voltage (mean, 218 V for children <3 years). The significantly lower stimulating voltage in their study was possible because of the low dose of propofol administered (100–135 μg/kg/hr, at times reduced to 35–65 μg/kg/hr) compared to 100–250 μg/kg/hr of propofol used by Fulkerson et al. [4].
A relatively low stimulating voltage (255.9 ± 36 V) and a high propofol dose (150–200 μg/kg/hr) and use of inhalational anesthetics could be the reason for not obtaining mMEPs in 6% of our children. Among the 6 children in whom base line MEPs were not obtained, 4 received inhalational agents and 2 received TIVA.
Compared to conventional mMEP techniques, use of double train (DT) stimulation [10] and post tetanic mMEP monitoring [11] yielded better responses in children.
mMEPs in TCS
Most of the literature on mMEPs in TCS pertains to studies in adults and older children where it has been shown to improve the safety of surgery and increase the radicality of excision of lipomas [12]. In children undergoing TCS, risk of postoperative neurological deficits significantly reduced from 9.4 to 2.9% with monitoring and long-term progression free percentage of children was significantly higher at 88.7% in those who had monitoring compared to 69.2% in those without monitoring [12].
MEPs were obtained in 94% of our children of whom 87% had baseline mMEPs. mMEPs were most consistently obtained from TA, soleus and EDB muscles, similar to the findings we reported earlier in older patients [13].
Baseline mMEPs are generally obtained better in patients with no deficits [14, 15]. Our finding that a high percentage of patients with motor deficits also had baseline mMEPs suggests that mMEP monitoring should be attempted even in those with motor dysfunction.
Effect of anesthesia on mMEPs
All inhalational anesthetic agents cause a dose-dependent reduction in amplitude of mMEP responses which vary among different agents. However, with higher-intensity stimuli and multi-pulse stimulation (3–5 pulse), volatile anesthetic (0.3–0.5 MAC) induced suppression can be eliminated [16,17,18,19]. In our study, responses were obtained in only 73% children using isoflurane while sevoflurane had a success rate of 91%.
Propofol administered in the dose range of 100–250 μg/kg/min in combination with opioids has the least interference with mMEP amplitude [4, 7, 16]. We used propofol (150–200 μg/kg/min) in combination with fentanyl (1–2 μg/kg/hr) to achieve a 93% success rate (38/41). A similar anesthetic approach was used by Yi et al. [9]. McIntyre et al. [6] obtained mMEPs in 86% children <2 years old, using a combination of propofol (100–200 μg/kg/min) and remifentanil (0.2–0.5 μg/kg/min). In our study, none of the children developed any complications related to TIVA using propofol.
Ketamine changes the alpha motor neuron excitability and increases the H reflex and thereby contributes to mMEP enhancement [16,17,18]. Ketamine is also used to augment the mMEP response in cases where the amplitude reduction could be due to the anesthetic fade effect. It can be used to help differentiate an anesthetic cause from a surgical cause for a drop in mMEP amplitudes.
Since opioids act through the opioid receptor and not through the GABA or NMDA receptors, they cause the least impact on IONM [16,17,18].
A study by Diener et al. [20] has shown that the anesthetic technique and co-morbidities have an additive effect on mMEP monitoring.
Our anesthetic regime
In healthy children, TIVA using propofol (150–200 μg/kg/hr) and fentanyl infusion (1–2 μg/kg/hr) is used. In children with low body weight for age, a balanced anesthetic regime consisting of low MAC (0.3–0.4 MAC) inhalational agent (sevoflurane/isoflurane), small dose of propofol (100–150 μg/kg/hr) and ketamine infusion (0.5 mg/kg/hr) is used. If the mMEP response is inadequate, sevoflurane is turned off and ketamine increased to 1 mg/kg/hr. TIVA or a combination of sevoflurane and ketamine was associated with the highest success rate of baseline mMEP responses.
Accuracy of mMEPs
An improvement in mMEPs may or may not result in an immediate clinical improvement, but often results in a long-term clinical improvement [21]. Out of the 36 children with improvement in mMEPs after surgery, two children (5.6%) showed immediate clinical improvement. We had reported similar findings in older children and adults undergoing TCS [21]. False positive cases, where there is drop in mMEPs with no postoperative deficits, have been reported by various authors [9, 22]. Our false positive rate of 3.6% is similar to the 4–4.5% reported in the literature [9, 22]. One of our children developed transient postoperative weakness though mMEPs were intact (false negative). A false negative result is obviously a worrying outcome but the cause for this is not known. False negative mMEP responses have been reported previously in spinal cord surgeries but are rare [10, 23]. Jin et al. [23] reported false negative results in two of 25 patients undergoing surgery for intramedullary spinal cord tumors. Both patients developed postoperative motor weakness, although mMEPs were retained throughout the surgery. They could not explain the false negative result.
The accuracy in our study of 96.8% is comparable to the accuracy of 95.7 and 95.5% reported in mMEP studies in children undergoing surgery for spinal and cranial pathologies [9, 22].
Conclusions
TcMEPs are safe in children ≤2 years of age with baseline recordings obtained in >85%. TIVA and balanced anesthesia with sevoflurane and ketamine are ideal for obtaining mMEP recordings. Diagnostic accuracy of mMEPs is high although occasional false positive and false negative results can occur.
References
Aydinlar EI, Dikmen PY, Kocak M, Baykan N, Seymen N, Ozek MM (2019) Intraoperative neuromonitoring of motor evoked potentials in infants undergoing surgery of the spine and spinal cord. J Clin Neurophysiol 36(1):60–66. https://doi.org/10.1097/WNP.0000000000000523
Şenköylü A, Zinnuroğlu M, Börçek A, Aktaş E, Güngör İ, Beyazova M (2017) Comparison of multimodal intraoperative neurophysiological monitoring efficacy in early childhood and school aged children undergoing spinal surgery. Acta Orthop Traumatol Turc 51(1):49–53. https://doi.org/10.1016/j.aott.2016.12.005
Lieberman JA, Lyon R, Feiner J, Diab M, Gregory GA (2006) The effect of age on motor evoked potentials in children under propofol/isoflurane anesthesia. Anesth Analg 103(2):316–321. https://doi.org/10.1213/01.ane.0000226142.15746.b2
Fulkerson DH, Satyan KB, Wilder LM, Riviello JJ, Stayer SA, Whitehead WE, Curry DJ, Dauser RC, Luerssen TG, Jea A (2011) Intraoperative monitoring of motor evoked potentials in very young children. J Neurosurg Pediatr 7(4):331–337. https://doi.org/10.3171/2011.1.PEDS10255
Vora TK, Girishan S, Moorthy RK, Rajshekhar V (2021) Early and long-term surgical outcomes in 109 children with lipomyelomeningocele. Childs Nerv Syst. https://doi.org/10.1007/s00381-020-05000-y Epub ahead of print
McIntyre IW, Francis L, McAuliffe JJ (2016) Transcranial motor evoked potentials are more readily acquired than somatosensory evoked potentials in children younger than 6 years. Anesth Analg 122(1):212–218. https://doi.org/10.1213/ANE.0000000000001044
Macdonald DB (2006) Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 20(5):347–377. https://doi.org/10.1007/s10877-006-9033-0
Sala F, Squintani G, Tramontano V, Arcaro C, Faccioli F, Mazza C (2013) Intraoperative neurophysiology in tethered cord surgery: techniques and results. Childs Nerv Syst 29(9):1611–1624. https://doi.org/10.1007/s00381-013-2188-3
Yi YG, Kim K, Shin HI et al (2019) Feasibility of intraoperative monitoring of motor evoked potentials obtained through transcranial electrical stimulation in infants younger than 3 months. J Neurosurg Pediatr 23(6):1–9. https://doi.org/10.3171/2019.1.PEDS18674
Journée HL, Polak HE, de Kleuver M, Langeloo DD, Postma AA (2004) Improved neuromonitoring during spinal surgery using double-train transcranial electrical stimulation. Med Biol Eng Comput 42(1):110–113. https://doi.org/10.1007/BF02351019
Hayashi H, Kawaguchi M, Yamamoto Y et al (2008) Evaluation of reliability of post-tetanic motor-evoked potential monitoring during spinal surgery under general anesthesia. Spine (Phila Pa 1976) 33(26):E994–E1000. https://doi.org/10.1097/BRS.0b013e318188adfc
Fekete G, Bognár L, Novák L (2019) Surgical treatment of tethered cord syndrome-comparing the results of surgeries with and without electrophysiological monitoring. Childs Nerv Syst 35(6):979–984. https://doi.org/10.1007/s00381-019-04129-9
Rajshekhar V, Velayutham P, Joseph M, Babu KS (2011) Factors predicting the feasibility of monitoring lower-limb muscle motor evoked potentials in patients undergoing excision of spinal cord tumors. J Neurosurg Spine 14(6):748–753. https://doi.org/10.3171/2011.1.SPINE10310
Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, Bricolo A (2006) Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery 58(6):1129–1143. https://doi.org/10.1227/01.NEU.0000215948.97195.58
El-Hawary R, Sucato DJ, Sparagana S et al (2006) Spinal cord monitoring in patients with spinal deformity and neural axis abnormalities: a comparison with adolescent idiopathic scoliosis patients. Spine (Phila Pa 1976) 31(19):E698–E706. https://doi.org/10.1097/01.brs.0000232707.98076.37
Lotto ML, Banoub M, Schubert A (2004) Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J Neurosurg Anesthesiol 16(1):32–42. https://doi.org/10.1097/00008506-200401000-00008
Sloan T (2010) Anesthesia and intraoperative neurophysiological monitoring in children. Childs Nerv Syst 26(2):227–235. https://doi.org/10.1007/s00381-009-1023-3
Bithal PK (2014) Anaesthetic considerations for evoked potentials monitoring. J Neuroanaesthesiol Crit Care 1(01):002–012
Inoue S, Kawaguchi M, Takashi S, Kakimoto M, Sakamoto T, Kitaguchi K, Furuya H, Morimoto T, Sakaki T (2002) Intraoperative monitoring of myogenic motor-evoked potentials from the external anal sphincter muscle to transcranial electrical stimulation. Spine (Phila Pa 1976) 27(21):E454–E459. https://doi.org/10.1097/00007632-200211010-00018
Deiner SG, Kwatra SG, Lin HM, Weisz DJ (2010) Patient characteristics and anesthetic technique are additive but not synergistic predictors of successful motor evoked potential monitoring. Anesth Analg 111(2):421–425. https://doi.org/10.1213/ANE.0b013e3181e41804
Pratheesh R, Babu KS, Rajshekhar V (2014) Improvement in intraoperative transcranial electrical motor-evoked potentials in tethered cord surgery: an analysis of 45 cases. Acta Neurochir 156(4):723–731. https://doi.org/10.1007/s00701-014-1999-7
Noonan KJ, Walker T, Feinberg JR, Nagel M, Didelot W, Lindseth R (2002) Factors related to false- versus true-positive neuromonitoring changes in adolescent idiopathic scoliosis surgery. Spine (Phila Pa 1976) 27(8):825–830. https://doi.org/10.1097/00007632-200204150-00009
Jin SH, Chung CK, Kim CH, Choi YD, Kwak G, Kim BE (2015) Multimodal intraoperative monitoring during intramedullary spinal cord tumor surgery. Acta Neurochir 157(12):2149–2155. https://doi.org/10.1007/s00701-015-2598-y
Author information
Authors and Affiliations
Contributions
The study conception, design and supervision were done by Vedantam Rajshekhar. Material preparation, data collection, and analysis were performed by Bijesh and Krothapalli Srinivasa Babu. The first draft of the manuscript was written by Bijesh, Mariappan Ramamani and Georgene Singh. Manuscript was reviewed and edited by Krothapalli Srinivasa Babu and Vedantam Rajshekhar. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nair, B.R., Ramamani, M., Singh, G. et al. Feasibility and diagnostic accuracy of intra-operative monitoring of motor evoked potentials in children <2 years of age undergoing tethered cord surgery: results in 100 children. Childs Nerv Syst 37, 2289–2298 (2021). https://doi.org/10.1007/s00381-021-05128-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05128-5