Abstract
Purpose
Proton beam therapy (PBT) is now well established for the treatment of certain pediatric brain tumors. The intrinsic properties of PBT are known to reduce long-term negative effects of photon radiotherapy (PRT). To better understand the intracranial effects of PBT, we analyzed the longitudinal imaging changes in a cohort of children with brain tumors treated by PBT with clinical and radiotherapy dose correlations.
Materials and methods
Retrospective imaging review of 46 patients from our hospital with brain tumors treated by PBT. The imaging findings were correlated with clinical and dose parameters.
Results
Imaging changes were assessed by reviewing serial magnetic resonance imaging (MRI) scans following PBT over a follow-up period ranging from 1 month to 7 years. Imaging changes were observed in 23 patients undergoing PBT and categorized as pseudoprogression (10 patients, 43%), white matter changes (6 patients, 23%), parenchymal atrophy (6 patients, 23%), and cerebral large vessel arteriopathy (5 patients, 25%). Three patients had more than one type of imaging change. Clinical symptoms attributable to PBT were observed in 13 (28%) patients.
Conclusion
In accordance with published literature, we found evidence of varied intracranial imaging changes in pediatric brain tumor patients treated with PBT. There was a higher incidence (10%) of large vessel cerebral arteriopathy in our cohort than previously described in the literature. Twenty-eight percent of patients had clinical sequelae as a result of these changes, particularly in the large vessel arteriopathy subgroup, arguing the need for angiographic and perfusion surveillance to pre-empt any morbidities and offer potential neuro-protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proton beam therapy (PBT) is now established as a treatment for certain pediatric brain tumors. Although PBT is not more effective in treatment of brain tumors, its potential benefits stem from the intrinsic properties of the radiation, such as absence of an exit dose, optimal dose distribution, and reduced radiation dose to adjacent normal tissue. This potentially reduces some of the negative long-term or delayed effects associated with photon radiotherapy (PRT) such as cognitive deficits, endocrine and vascular abnormalities, and development of secondary malignancies [1,2,3].
As treatment with PBT becomes commonplace, the incidence of treatment-related effects, both clinical and radiological, has also become apparent.
In this paper, we present the findings of a descriptive study highlighting the imaging changes in a cohort of children with different types of brain tumors treated with PBT. The imaging findings have been correlated with clinical and dose parameters.
We anticipate that this will help inform clinicians, oncologists, and neuroradiologists of the spectrum of imaging changes associated with PBT and their potential implications.
Methods
We retrospectively interrogated the neuro-oncology database of our hospital for all children with intracranial tumors treated with single-dose PBT between 2008 and 2016. All children also underwent adjuvant surgery and/or chemotherapy as a part of institutional and nationally approved treatment protocols (Table 1). Institutional board approval was obtained for this review (audit registration number 2089).
Our inclusion criteria included newly diagnosed primary brain tumors, a single course of PBT, and no PRT. Extracranial tumors were excluded. Of 56 patients undergoing PBT, 46 were selected as per the inclusion criteria.
All the patients received PBT in external centers where standardized dose-calculating algorithms were used.
Neuro-oncology protocol MRI sequences (comprising a minimum of axial and coronal T2-weighted spin echo, axial T2-FLAIR, axial diffusion–weighted imaging, pre- and post-contrast axial and coronal T1-weighted spin echo) were acquired in all patients. Imaging was performed using 1.5 T Siemens (Erlangen, Germany) Avanto scanners.
Two pediatric neuroradiologists conducted a consensus read of the pre-treatment and serial post-treatment imaging. The follow-up time period for imaging ranged from 1 to 87 months.
The adopted parameters for defining pseudoprogression were an increase in the tumoral volume or new abnormal enhancement, an increase in tumor size or new abnormal enhancement, occurring 3–4 months after the completion of radiotherapy, which subsequently resolved without treatment. This definition is commensurate with the data in the literature.
Results
Imaging changes were noted in 23 (50%) patients (13M:10F). The age range of these patients was 4–13 years. The imaging changes were sub-classified as white matter changes, pseudoprogression, cerebral arteriopathy, and parenchymal atrophy (Fig. 1). More than one imaging change was identified in three patients. The imaging changes were correlated with tumor type and location, clinical course of the patient, and the PBT radiation dosage (Table 1).
White matter changes
White matter changes were observed in six patients (26%) and defined as signal abnormality, typically hyperintense on T2 and T2-FLAIR-weighted imaging, in the cerebral or cerebellar white matter (Fig. 2). Changes were only included in this subcategory if there was no associated abnormal enhancement or corresponding changes on diffusion-weighted imaging. The white matter changes were assessed qualitatively and were transient in all but one case.
In two patients, the white matter changes were distant (at least 3 cm) from the area of irradiation. In the remaining case, the changes were confined to the region of the irradiated tumor.
The white matter changes were not considered to be related to natural evolution of the tumor or surgical treatment (such as drainage of tumoral cysts or direct surgical manipulation of tissue) on the basis of the time interval since surgery. Apart from transiently increased headaches, there was no relevant correlation with clinical symptomatology.
Large vessel cerebral arteriopathy
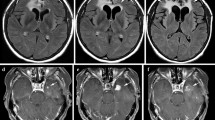
Definite, and to a certain extent, progressive macrovascular cerebral arteriopathy (vessel narrowing and irregularity) was seen in five (22%) patients with coexistent parenchymal ischemic changes in some cases (Figs. 3 and 4).
In a few cases, there were territorial arterial infarcts, with other potential causes of infarction having been excluded. We confirmed necrosis on biopsy in one patient. The case was biopsied as there was discrepancy among the clinical team if the lesions represented true progression or not, with indeterminate results on advanced imaging (MR perfusion); being a posterior fossa lesion, MR spectroscopy also was inconclusive because of technical limitations.
Clinical signs and symptoms around arteriopathy were a spread of children presenting with obvious stroke like manifestations, and those in which the arteriopathy was asymptomatic, or even transient. We do appreciate that some of the patients with craniopharyngiomas had surgical interventional procedures, which could be a confounding factor especially when assessing atrophy.
Pseudoprogression
The adopted parameters for defining pseudoprogression were an increase in tumor size or new abnormal enhancement, occurring 3–4 months after the completion of radiotherapy, which subsequently resolved without treatment [4,5,6,7,8]. Pseudoprogression was observed in 10 patients (43%).
For simplification, we have included those changes that may be considered as radiation necrosis in this category, as radiation necrosis is a histological diagnosis and conventional imaging does not easily discriminate between the two entities(Figs. 5 and 6) [9]. Most of our cohort did not have advanced MR imaging such as perfusion and spectroscopy.
Some of these patients also showed concomitant changes described in other categories. For example, the vascular changes in a case of a posterior fossa anaplastic ependymoma were also accompanied by presumed radionecrosis in the adjacent brain parenchyma. Tissue obtained from the area of signal abnormality due to diagnostic uncertainty in the latter case revealed scar tissue.
Clinically, the patients were not severely symptomatic, and the changes if any resolved along with resolution on imaging; thus, there was good correlation between neuroimaging findings and clinical presentation.
Example of CA and radionecrosis type changes. Self-resolving ischemic changes in patient with posterior fossa anaplastic ependymoma. Axial FLAIR (a, b), axial DWI (c), and axial post-contrast T1W (d) sequences showing progressive abnormal signal change, diffusion restriction, and enhancement, respectively, in pons and R MCP (biopsy confirmed necrotic tissue) (Fig. 5). Axial T2W and DWI sequences (e, f) showing resolution of changes after 1 year
Histology for radionecrosis. a Hematoxylin and eosin (HE) stain showing reactive gliotic brain tissue containing eosinophilic spheroids. b Staining for phosphorylated high-molecular weight neurofilament (NF) showing that some of the spheroids were positive (arrow) and some negative (arrowhead). Hyalinization of the vessel walls with histological evidence of tumor (scale bar 25 μm)
Parenchymal atrophy
Generalized atrophy was noted in six (26%) cases. Specific pituitary volume was observed loss in two patients with craniopharyngiomas (Table 2). Atrophy was qualitatively judged by consensus opinion between the two neuroradiologists as the imaging set did not always include a volumetric T1- or T2-weighted sequence.
The criteria for atrophy were change in ventricle size as well as enlargement of the subarachnoid spaces [9]. Atrophy did not correlate with obvious clinical (general growth/cognitive) deterioration in our cohort although dedicated cognitive assessment data were not available or the follow-up not adequate to evaluate for cognitive changes appropriately.
Types and chronology of imaging changes
The onset of imaging changes ranged from 1 month in cases of white matter changes and pseudoprogression to 65 months in a case of pseudoprogression and radiation-induced arteriopathy.
The duration of changes ranged from 1 to 61 months. In the cases of arteriopathy and parenchymal atrophy, the changes were irreversible. In the remaining two categories, there were some instances of transient change, as per the definition of the categories. The chronological parameters for these categories are as shown in the below bar diagrams (Figs. 7 and 8).
There was no clear relationship between imaging change type, tumor type, and imaging change chronology.
Radiation doses
The radiation dose was recorded in 21 patients; dosages could not be obtained for 2 patients. The doses ranged from 50 to 60 Gy (RBE). Some posterior fossa tumors received localized boost radiation doses with no individual-fractionated dose exceeding 2 Gy.
Correlation of imaging changes to clinical symptoms
Clinical data were available for 22 of the 23 patients who had imaging changes. Clinical symptoms were noted in 13 patients at time of the imaging changes.
Although the clinical symptoms in most of this cohort were transient and self-resolving, there were significant morbidities in a few patients. Patients with radiation-induced arteriopathy had worse and permanent neurological deficits. Examples of these included a patient who required carotid revascularization for cerebral arteriopathy. The patient was left with residual neurological deficits. Another patient who presented with acute cranial nerve palsies and focal weakness showed significantly delayed presumed radiation necrosis-type changes with arteriopathy. A further patient who had pseudoprogression-type changes in the brainstem had diplopia requiring corrective lenses.
Other presentations included focal seizures in one patient with an ependymoma with cerebral pseudoprogression-type changes requiring treatment with anticonvulsants. Generalized weakness, problems with walking, and vomiting were seen in a patient with posterior fossa volume loss requiring neuro-rehabilitation.
The symptoms in the remaining patients were mostly comprised of transient headaches and episodic dizziness.
Discussion
The effects of treatment with PBT have been of increasing interest for some time. Previous studies have looked at imaging changes in isolation in certain specific tumor groups and at certain individual clinical aspects. Most studies have also been limited by relatively short follow-up times, notable exceptions being a study by McGovern et al. looking at toxicities after PBT in patients with AT/RT, following up patients up to 53 months [10].
There are also studies examining the effects of PBT in isolation, as well as comparing them with PRT cohorts [11, 12]. Sabin et al. looked at imaging changes in eight very young children with a mean age of 1.8 years with a variety of brain tumors. The changes described were those of pseudoprogression with transient neurological symptoms [13]. Gunther et al. found post-radiation imaging changes in pediatric intracranial ependymoma patients to be more common when treated with PBT, compared with photon-based intensity-modulated radiotherapy (IMRT) [14]. Uh et al. looked at radiation dose effects on the structural integrity of cerebral WM using DTI data in a series of 51 craniopharyngioma patients treated with surgery and PBT, with findings indicating transient reduction in FA values, and greater reductions with higher doses [11].
Our study had a much wider range of tumor types, as well as longer follow-up times, up to 87 months. As expected, the treatment histories were also more complex.
Our study showed the bulk of changes to include white matter signal changes and pseudoprogression. Given the recognized overlap between the definitions of pseudoprogression and radiation-induced change [11, 14, 15], we chose to include changes that other authors may describe as radiation necrosis as part of the former term.
Pulsifer et al. studied the cognitive and adaptive outcomes following PBT in a series of 155 patients with a mean follow-up of 3.6 years, with encouraging results; a higher intelligence quotient (IQ) decline was shown in patients under 6 years of age with craniospinal irradiation while other patients showed only a slight IQ decline [16]. In our cohort, one patient with an astrocytoma with WM changes in the brainstem showed transient acute behavioral changes, falls, and worsening headaches. He was also diagnosed with autism during this period. We did not observe any obvious cognitive decline within the study period in our cohort, though this may occur later in life and warrants further long-term evaluation, which is currently underway.
Cerebral arteriopathy
Radiation-induced cerebral arteriopathy is a recognized phenomenon in patients who have undergone PRT. Until recently, it was not reported widely in patients undergoing PBT [17, 18]. Traditionally, authors have suggested that one of the important positives while using PBT to treat tumors such as sellar craniopharyngiomas is the potential to reduce radiation dose to the circle of Willis, which may reduce the risk of future cerebrovascular complications [19]. However, five patients in our cohort (22%) had large vessel arteriopathy, including two with suprasellar craniopharyngiomas. Three of these patients developed focal parenchymal ischemic lesions and one patient required carotid revascularization. These patients had been assessed for and cleared of other risk factors of stroke, such as hypercoagulable states, autoimmune diseases, and infection. Previous surgery may be an additional confounding factor in some cases.
The pathophysiology of cerebral large vessel arteriopathy still remains unclear in PBT patients. In PRT subgroups, hallmarks of large vessel arteriopathy are intimal thickening and medial necrosis. We are awaiting vessel wall histopathological analysis in our PBT cohort to confirm if the changes are similar. Some authors have postulated the potential of increased risks of developing vasculopathy with larger doses of radiation [20]; this however does not apply to our subgroup, with standard radiation doses.
Secondary malignancies
No radiation-induced tumors were noted in our patient cohort. In the sole patient who underwent a biopsy, histopathology confirmed necrotic tissue with no tumor.
Development of changes distant to field of irradiation
One of the important rationales for PBT is its superior dose distribution compared with PRT suggesting sparing of the normal tissue from the adverse effects of the radiation. Redial et al. reported a case of delayed onset acute remote demyelination after focal PBT for an optic nerve meningioma [21]. We also noticed white matter changes distant to the area of irradiation in a few cases. These were however transient with no clinical deterioration.
The etiology of similar non-contiguous changes in patients undergoing PRT has been suggested as a vascular insult secondary to the combination of PRT with more potent antiangiogenic therapy [22]. While these have not been reported in PBT patients so far, and our patient cohort did not receive any antiangiogenic therapy, we postulate that a microvascular ischemic process would be the most likely explanation in these cases.
Radiation doses
Although correlation of radiation doses with imaging changes was not performed for individual cases, given that the total radiation dose as well as individual fractionated doses in our cohort was well below the levels associated with radiation-induced adverse effects [12], we did not feel that cumulative radiation doses were a causative factor behind the imaging changes.
Atrophy
The importance of parenchymal volume loss in the maturing young brain cannot be overemphasized, in spite of no formal clinical correlate, and the absence of a control group in our study. We also realize that previous surgery can be a confounding factor in our cohort, especially in the case of the craniopharyngiomas. Further comparison with parenchymal volumes in purely surgically treated patient cohorts would be needed to more accurately assess the effects of PBT on brain and pituitary volumes.
Role of chemotherapy
Of the patients showing imaging changes, three had chemotherapy before PBT. All of these were low grade astrocytic tumors. The imaging changes comprised of white matter changes, pseudoprogression, and one case of cerebral arteriopathy. While pseudoprogression can be associated with combined radio-chemotherapy, we did not feel that the cerebral arteriopathy was etiologically linked to the chemotherapy. This assumption was based on the description of the chemotherapy-induced brain changes in literature which were different from those in our cohort [13].
Limitations
Our study does have certain limitations. Only conventional imaging sequences as available in this retrospective cohort were analyzed. We are aware that complementary imaging sequences such as MR perfusion, MR spectroscopy, and susceptibility-weighted imaging would be useful adjuncts for the assessment of treatment-related changes, and these have been prospectively included in our imaging protocol. As we are a tertiary referral center, not all cases followed up elsewhere had volumetric data available for quantitative assessments of atrophy or tumor volume change. The strength of evidence can be further improved with further follow-up of this cohort, and additional patients, specifically in relation to their cognitive outcomes.
The limitations of our study also include a degree of overlap between pseudoprogression, radionecrosis, and white matter changes; this is in part due to the evolving understanding of the pathophysiology of these changes, and we felt that a broader classification would better serve the scope of this descriptive study.
Other factors such as effects of previous surgery could be considered as confounding factors in some categories; however, we feel that these effects were not significant in our patient cohort.
Conclusion
Our findings reconfirmed the occurrence of imaging changes in patients who were treated with PBT; some of these changes were in concordance with those reported by previous authors, such as pseudoprogression and radiation necrosis [6, 23, 24] However, we also found incidence of large vessel progressive cerebral arteriopathy as recently described with PBT, more than previously reported by Kralik et al. [25]. This argues the need for dedicated angiographic imaging as part of routine tumor surveillance imaging in post-PBT patients, to detect these changes at an early stage, before the onset of debilitating morbidities. The role of advanced imaging such as vessel wall imaging has been postulated by other authors [18], which could be considered given increasing documentation of macrovascular arteriopathy, as well as perfusion imaging and MR spectroscopy (in anatomically suitable lesions).
We also noted the appearance of transient white matter changes distant to the region of irradiation; along with our findings of large vessel arteriopathy, we feel that this does challenge the existing concepts of PBT delivering radiation within a defined radiation track length, with virtually no dose beyond the intended target.
Some previous authors perceived the imaging changes to have minimal clinical significance, while this was true for the majority of our cohort, we did have a few patients who were left with significant residual morbidities as a result of post-treatment changes, as well as patients who required major revascularization surgery.
Our findings stress the need for continued close follow-up of pediatric patient populations treated by PBT to further document radiological and clinical changes, thus enabling us to assess the long-term effects and true benefits of this treatment method.
Abbreviations
- AT/RT:
-
Atypical teratoid rhabdoid tumor
- CA:
-
Cerebral arteriopathy
- CT:
-
Chemotherapy
- FA:
-
Fractional anisotropy
- HA:
-
Headache
- LGG:
-
Low grade glioma
- MB:
-
Medulloblastoma
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- NF:
-
Neurofibromatosis;
- OPG:
-
Optic pathway glioma
- PBT:
-
Proton beam therapy
- PRT:
-
Photon radiotherapy
- RBE:
-
Relative biological effectiveness
- SWI:
-
Susceptibility weighted imaging
- WM:
-
White matter
References
Halperin EC, Brady LW, Perez CA, Wazer DE (2013) Perez & Brady’s principles and practice of radiation oncology. Lippincott Williams & Wilkins
Gondi V, Yock TI, Mehta MP (2016) Proton therapy for paediatric CNS tumours — improving treatment-related outcomes. Nature Reviews Neurology 12:334–345
Sands SA (2016) Proton beam radiation therapy: the future may prove brighter for pediatric patients with brain tumors. J. Clin. Oncol. 34:1024–1026
Parvez K, Parvez A, Zadeh G (2014) The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15:11832–11846
Wang S, Martinez-Lage M, Sakai Y et al (2016) Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. AJNR Am J Neuroradiol 37:28–36
Hygino da Cruz LC Jr, Rodriguez I, Domingues RC et al (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 32:1978–1985
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22:633–638
Thust S, van den Bent MJ, Smits M (2018) Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 48:571–589
Zivadinov R, Bergsland N, Korn JR et al (2017) Feasibility of brain atrophy measurement in clinical routine without prior standardization of the MRI protocol: results from MS-MRIUS, a longitudinal observational, multicenter real-world outcome study in patients with relapsing-remitting MS. AJNR Am J Neuroradiology 39:289–295
McGovern SL, Okcu MF, Munsell MF et al (2014) Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys 90:1143–1152
Uh J, Merchant TE, Li Y et al (2015) Effects of surgery and proton therapy on cerebral white matter of craniopharyngioma patients. International Journal of Radiation Oncology Biology Physics 93:64–71
Lundkvist J, Ekman M, Ericsson SR et al (2005) Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncologica 44:850–861
Sabin ND, Merchant TE, Harreld JH et al (2013) Imaging changes in very young children with brain tumors treated with proton therapy and chemotherapy. American Journal of Neuroradiology 34:446–450
Gunther JR, Sato M, Chintagumpala M et al (2015) Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. International Journal of Radiation Oncology Biology Physics 93:54–63
Giantsoudi D, Sethi RV, Yeap BY et al (2016) Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: LET and RBE associations for areas of injury. International Journal of Radiation Oncolog Biology Physics 95:287–296
Pulsifer MB, Duncanson H, Grieco J et al (2018) Cognitive and adaptive outcomes after proton radiation for pediatric patients with brain tumors. Int J Radiat Oncol Biol Phys 102:391–398
Dynlacht JR (2011) Human radiation injury, edited by Dennis C. Shrieve and Jay S. Loeffler. Radiation Research 176:273–274
Nordstrom M, Felton E, Sear K et al (2018) Large vessel arteriopathy after cranial radiation therapy in pediatric brain tumor survivors. Journal of Child Neurology 33:359–366
Conroy R, Gomes L, Owen C et al (2015) Clinical equipoise: protons and the child with craniopharyngioma. Journal of Medical Imaging and Radiation Oncology 59:379–385
Desai SS, Paulino AC, Mai WY, Teh BS (2006) Radiation-induced moyamoya syndrome. International Journal of Radiation Oncology Biology Physics 65:1222–1227
Redjal N, Agarwalla PK, Dietrich J et al (2015) Remote acute demyelination after focal proton radiation therapy for optic nerve meningioma. J Clin Neurosci 22:1367–1369
Patay Z, Merchant TE, Nguyen R et al (2017) Treatment-related non-contiguous radiologic changes in children with diffuse intrinsic pontine glioma treated with expanded irradiation fields and antiangiogenic therapy. Int J Radiat Oncol Biol Phys 99:1295–1305
Meyzer C, Dhermain F, Ducreux D et al (2010) A case report of pseudoprogression followed by complete remission after proton-beam irradiation for a low-grade glioma in a teenager: the value of dynamic contrast-enhanced MRI. Radiation Oncology 5:9
Kralik SF, Ho CY, Finke W et al (2015) Radiation necrosis in pediatric patients with brain tumors treated with proton radiotherapy. AJNR Am J Neuroradiol 36:1572–1578
Kralik SF, Watson GA, Shih C-S et al (2017) Radiation-induced large vessel cerebral vasculopathy in pediatric patients with brain tumors treated with proton radiation therapy. Int J Radiat Oncol Biol Phys 99:817–824
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhattacharya, D., Chhabda, S., Lakshmanan, R. et al. Spectrum of neuroimaging findings post-proton beam therapy in a large pediatric cohort. Childs Nerv Syst 37, 435–446 (2021). https://doi.org/10.1007/s00381-020-04819-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04819-9