Abstract
This review looks at the advances in the surgical technique, selective dorsal rhizotomy, used for the management of spasticity in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
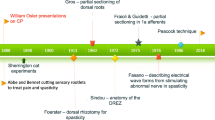
In the mid-1980s, the selective dorsal rhizotomy (SDR) was introduced to North America for the treatment of spasticity and, in particular, the treatment of children with spastic cerebral palsy (CP). In the late 1980s, I was asked to write a review of its history and its use [1]. At the time the favored technique was based on Peacock’s [2, 3]. It consisted of a L2 through L5 laminectomies wide enough to view the nerve roots as they exited the dura, separation of the sensory from the motor nerve root at each targeted level then electrical stimulation of the L2 through S1 sensory roots bilaterally. Interpretation of the responses was based on Fasano’s manuscript that described an normal root response to an electrical stimulus train (1 stimulus/s) as causing contraction of a single muscle or muscle group that continued in a one-for-one pattern [4]. As the frequency of stimuli in the train was increased, the observed muscle contraction rapidly lessened in duration and ceased when the train’s frequency of stimuli went above 20 Hz. He also described a normal root’s response as “The responding muscle groups are always the same, for each root being examined, whatever stimulation frequency is employed.” He observed that the threshold for stimulation of normal nerve roots was between 0.1 and 0.5 V. Fasano deemed a nerve root as being abnormal when its response to stimulation varied from the above description.
Peacock’s presentation of his technique and his reports on his CP patients’ outcome rapidly gained attention in North America, both by its pediatric neurosurgeons and the public at large. Much controversy was generated in the medical field over the procedure and its efficacy. Surgeons employing the technique were compelled to report their results in an increasingly stringent manor. The result has been an evolution in the methods used both surgically and for assessing the patients undergoing SDR. This review will focus on this evolution and how it has affected patient selection for treatment with SDRs, the tools used for their assessment, the surgical technique, and some of the complications that have been driving this evolution.
Candidate selection
In his 1987 report on using SDR to treat “cerebral palsy spasticity,” Peacock concluded that children with pure spasticity that predominantly involves the legs benefited most from the procedure and this became his ideal candidate [5]. The paper also reported that “…athetoid and ataxic patients should not undergo this form of surgery unless there is considerable amount of coexisting spasticity.” Great attention was placed on clearly defining spasticity as described by Lance (“…a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) with exaggerated tendon jerks, resulting from hyperexcitability of the stretch reflex, as one component of the upper motor neuron syndrome.”) [6]. Peacock’s subsequent reports focused on the results of using SDR to treat spastic diplegics. This, coupled with the introduction of intrathecal baclofen, narrowed the application of SDR to children in most centers by the late 1990s.
Early on investigators during presentations and in informal discussion antidotally mentioned that they were seeing better function in their patients’ upper extremities, questioning if SDR had wider applicability. Indeed, Peacock reported seeing improvement in 19 0f 28 spastic diplegic children with abnormal fine motor ability in their upper extremities but good trunk control and some ability to locomote. Additionally, he reported performing SDRs on 11 spastic quadriplegic children with difficulties in upper extremity function and an inability to voluntarily move finding all to benefit from the procedure. In 1998 Loewen et al. using validated assessment tools for upper extremity function and activities of daily living reported statistically significant improvement in functional use of the arms and in activities of daily living [7]. Their study group included 22 spastic quadriplegics. Others reported improvements in muscle tone in the upper extremities in their patients undergoing SDRs [8,9,10,11].
More recently, as a consequence of these findings and a realization of the problems in using intrathecal baclofen in children, reports have begun to appear specifically addressing the use of SDR in spastic quadriplegics. Morota reported on 3 spastic quadriplegics, all with severe spastic quadriplegics, who he performed SDR on [12]. He found that all had significant drops in their lower extremity mean Ashworth scores (3.5 → 1.4, 4.5 → 1.2, and 4.8 → 1.3). One of the three experienced a mild improvement in Gross Motor Function Measure (GMFM) scores, and all were free of severe opisthotonos posturing after their surgery. All the patients’ families were pleased with the result describing an easing in the burden of caring for their family member. Ailon et al. reported in 2015 on their patients’ long-term (> 10 years post SDR) results, and included in this report were seventeen children whose Gross Motor Function Classification Scale [13] (GMFCS) was level 4 and three who were GMFCS level 5 [14]. When lumped together, they found that the group dropped their mean Ashworth score by 1.4 at 6 months to 5 years and another 0.5 points when examined ≥ 10 years. There was an initial improvement in function in this group, but by 10 years, their functional scores were lower than those obtained preoperatively. Ingale reported on an interesting group of spastic quadriplegic children who initially were treated with intrathecal baclofen and whose families chose to switch their treatment to a SDR either because the pump became infected (3 children) or at the time of battery exhaustion of the pump they expressed displeasure with the treatment and requested an alternative (7 children) [15]. Three of these children were GMFCS level 4, and seven were GMFCS level 5. The stated goal for these children was to decrease their spasticity to ease in their care by their families. They found that there was a drop in the mean Ashworth score of 2.4 (pre-op to 8 months 3.6 → 1.3) but that only half of the patients maintained this improvement when examined at 14 months. Two of the GMFCS level 4 children gained the ability to quadruped crawl, and two of the GMFCS level 5 children gained the ability to independently sit. 9/10 families felt that the SDR had been beneficial. Buizer et al. reported their early experience in treating severely involved children with a history of CP or other congenital conditions resulting in spasticity. In their group of 18 patients were 15 with CP [16]. They found that all experienced a decrease in the number of spastic muscles after their rhizotomy. Many, however, still presented problems in daily care to their families and had persisting pain. Five of these CP children developed evidence of dystonia after their SDR in spite of preoperative MRIs showing no evidence of basil ganglia abnormalities. They concluded that while SDR is an alternative to be considered in treating such children, but caution was needed to discern any evidence of dystonia. They also felt that their results needed to be considered with caution owing to their lack of long-term follow-up.
Gump et al. published a review of the literature for SDR used to treat individuals with spasticity due to conditions other than CP [17]. They found reports on its being used for 74 patients with multiple sclerosis, 35 patients with spinal cord injuries, 9 with neurodegenerative disease including amyotrophic lateral sclerosis, 8 traumatic brain injuries, 2 congenital brain malformations, 2 hemorrhagic stroke, 2 ischemic strokes, 2 hydrocephalus, 2 hypoxic brain injuries during heart attacks, 2 near drownings, 2 transverse myelitis, 1 brain tumor, 1 hereditary spastic paraparesis, 1 meningitis, 1 myelomeningocele, and 1 myelopathy of an unspecified nature for a total of 145 non-CP spastic patients receiving a SDR. They found difficulty in analyzing the results because the standardized assessment tools commonly used for patients with CP had not been employed and because of the lack of description of long-term outcome. The reports they did review seemed to indicate a high success rate in treating the spasticity present and mixed results in improving function. In 2014 Reynolds et al. added three patients with severe spasticity due to a spinal cord injury [18]. Two of these patients experience good relief short term from their spasticity (mean Ashworth scores of 1 for each), while the third had return of his spasticity within 6 months and went on to have intrathecal baclofen therapy. In the same year, Li et al. added 4 patients with spastic hereditary paraparesis [19]. For this group, they found that the mean Ashworth score preoperatively averaged 3.8 ± 0.3 and that this average dropped significantly to 1.8 ± 0.2 at the 2-year follow-up examination. In their conclusion, they emphasized that their results were preliminary and needed long-term validation.
While the studies above and their mixed results emphasize our continued lack of understanding of the neurophysiology of spasticity and the precise neural circuitry that SDR is targeting for lesioning, they support a continued exploration of the efficacy for SDR in treating individuals with conditions other than spastic diplegic CP. This exploration, however, should include attempts at better understanding how such patients differ physiologically from the patient with spastic diplegic CP for which the efficacy of SDR is now well proven.
Assessment tools
Strength examination
Children with spastic diplegia (symmetrically involvement of the leg muscles with spasticity and minimal spasticity in the arms) have traditionally been the preferred patients for treatment with a SDR. The vast majority of such children will be ambulatory, distributed in the GMFCS levels 1 through 3. Historically, there was great concern about the degree children with spastic diplegia incorporate the spasticity in their leg muscles into the forces being used to stand and walk. In another words, is there sufficient underlying muscle strength to maintain the functions of standing and walking after the leg spasticity is removed? In his first manuscript about outcome of South African patients who had undergone SDR, Peacock described 3 patients who could stand using extreme knee extensor spasticity but lost that ability after undergoing SDR [2]. In his summary, he warned that when “…voluntary power is poor spasticity may be useful in helping the patient to maintain the standing position, and its relief is then questionable.” The therapist involved in patient selection for his group described a simple examination (squat-to-stand test) to differentiate strength from spasticity in the leg’s antigravity muscles [20]. It required the child to rise from a deep squatted position to full standing and then returning to a deep squat 7 times in succession using only the leg’s muscle strength (i.e., the child could not use the arms to pull to stand). The examiner was allowed to provide assistance in balance during the maneuver. This test allowed the examiner to evaluate the child’s ability to isolate and use the volitional strength of the leg to weight bear. Others heeded his warning and adopted this test to identify children at risk for losing the ability to stand if their spasticity were to be eliminated in the antigravity muscles of the legs [1, 21,22,23].
More recently there have been reports of utilizing gait analysis to differentiate volitional muscle strength from spasticity [24, 25]. Roberts found that a child with good muscle strength but “bad” spasticity tended to have a gait pattern that was repetitive without variation whereas a child with a variable gait pattern had weakness, poor motor control, or some other muscle disorder [24].
Tone examination
As noted above in the discussion about candidate selection, the young child with spastic diplegic cerebral palsy has traditionally been the preferred candidate for SDR. Broadening candidates to include spastic quadriplegia was explored early but with experience most groups moved to confine application of SDR to spastic diplegics as their experience grew [23]. A dogma developed that children with quadriplegic cerebral palsy were best avoided when selecting candidates for SDR. The difficultly with this is there is no precise definition of quadriplegic cerebral palsy and the condition has a spectrum of involvement of the upper extremities [26]. More recently, there has been a trend to first categorize children not as diplegic or quadriplegic but rather to use the GMFCS [13]. In doing so, children who have involvement of their upper extremities tend to distribute themselves into GMFCS levels 3 through 5. As a result, more mildly involved spastic quadriplegics are being included in patient populations undergoing SDR to improve motor function, and severely involved quadriplegics are receiving SDRs to ease in their care and/or provide pain relief [16, 27,28,29,30]. This stratification of quadriplegics based on their functional capabilities has allowed for a more discerning analysis of the applicability of SDR for treating spastic quadriplegic CP.
The trend of using functional classification in selection of treatments for children with cerebral palsy is undergoing further refinement. A recent paper by Schwartz et al. argues for assessing motor control over simple measures of single muscle and joint elements such as strength, tone, or mobility [31]. They argue that assessing such single elements only allows for an understanding of the “status quo” and that understanding a patient’s ability to control movement will perhaps allow for improvement in predicting outcome from a treatment, particularly in a child who has ambulator abilities at the time of considering a treatment.
Function examination
Programs developed to provide SDR treatment to children with disabling spasticity in the late 1980s and early 1990s most sought to document their patient’s functional abilities using tools developed in-house. Most sought to document how well the child could transition through the motor positions important in developing the ability to get from lying on the floor to standing. Many such tools broke the overall movement of lying to standing into stages such as moving from lying to hands and knee crawling position, then to kneeling, then to ½ kneeling and finally to stand. These tools graded the child’s quality of getting to each position as well as how well they looked while holding each position. While these in-house tools proved useful for tracking a treatment’s impact on any given patient for the center providing the care, they were not as useful in reporting overall results to colleagues at meetings or in scientific communication. In 1987 Russell et al. first published a tool developed to evaluate patients’ response to physical therapy, the Gross Motor Function Measure (GMFM) [32]. The tool consisted of 85 “items” or tasks that a 5-year-old child with normal motor abilities would be expected capable of completing. The items were grouped into 5 dimensions of body positions: (1) lying and rolling; (2) crawling and kneeling; (3) sitting; (4) standing; and (5) walking, running, and stair climbing. Each task was scored on a 4-point scale with 0 = cannot do, 1 = initiates, 2 = partially completes, and 3 = completes. This tool gained widespread respect among physical therapists in the early 1990s owing to the extent its developers had gone to ensure the tool’s sensitivity to documenting change in function and its intra-observer/inter-observer reliability. Concern arose as the GMFM-88 became widely used that it was most sensitive to changes in function in moderately involved children and that investigators had begun testing using only items contained in the dimensions felt appropriate for their study group. To increase the sensitivity of the GMFM and to accommodate how the tool was being used, the developers eliminated 22 of the GMFM-88’s items, introducing the GMFM-66 [33]. These two tools have been widely used to document change in motor function for many treatments used on children with CP including SDR [29,30,31,32,33,34]. The GMFM has become the gold standard for documenting change in the functioning of the motor system domain of the ICF resulting from a therapy being studied.
3D gait analysis has been available for several decades, and the need for its use has sparked much controversy [34]. It may, however, offer tools that would be helpful for differentiating patients in GMFCS level 3 who would be benefitted by undergoing a SDR from those who would not. Wong found that children who exhibited co-contraction of distal musculature when contracting the quadriceps had improved ambulation after SDR while those showing diffuse co-contraction did not [30]. Perhaps more important when considering level 3 children is whether or not they have sufficient control over the patterns of muscle contraction required for walking, i.e., sufficient muscle synergy. Cappellini et al. have shown that locomotion consists of two centrally controlled motor programs, one that organizes the pattern of muscle contractions during stance phase in the legs and the other during the swing phase [35]. These programs vary with the speed of locomotion, both in the timing of contraction for each involved muscle and in the strength of the contraction. Schwartz et al. have described a 3D gait analysis test for dynamic motor control (DMC), the so-called Walk-DMC [31]. This test seeks to quantify variance from the expected norm for muscle contraction patterns during the gait cycle. By analyzing muscle contraction, patterns during the gait cycle dystonia as well as spasticity can be recognized [30]. Such applications of gait analysis could prove a useful adjunct in considering GMFCS level 4 children where the presence of dystonia might sway one to consider intrathecal baclofen over the SDR for treatment. Using strategies such as this, we may be able to differentiate patients whose activation patterns predict insufficient control over their musculature to derive benefit from relief from their spasticity.
The impact of SDR on a child’s ability to engage in desired activities is important to consider, if for no other reason than to justify its use. There are tools that have been developed and are now validated for documenting changes in this ability. The two most widely used for children are the PEDI (the Pediatric Evaluation of Disability Inventory) and the WeeFIM (the Functional Independence Measure for Children) [36, 37]. The PEDI measures a child’s functional ability, his or her level of functional independence, and the degree of modifications need for this ability. It describes a child’s abilities for self-care, mobility, and social function in terms of his or her functional ability, the degree of caregiver support required, and modifications needed (i.e., none, child oriented, rehabilitation equipment, or extensive modifications). It contains 197 assessment questions that can be answered by parents, caregivers, or therapists in about 20–60 min, depending on the experience level of the individual scoring the questions. It is validated for use in children ages 0 to 8 years of age. The WeeFIM measures a child’s functional abilities in three domains: self-care, mobility, and cognition. It contains 18 items that are scored on a 7-point scale with 6 and 7 describing independence with or without modification of task (e.g., +/− using an assistive device) and 1–5 lessening levels of caregiver assistance to complete a task. It can be completed in less than 20 min and can be done by parents, caregivers, or therapists who have received instruction in use of this tool (formal course and online test for credentialing). The PEDI and WeeFIM differ in their emphasis with the former being stronger in assessing the social aspects of the child’s disability and the later the medical. Additionally, the WeeFIM is much more expensive, costing almost 20 times more than the PEDI to use on a child in the outpatient community. The PEDI has been used to assess outcome in children undergoing SDR by three groups and the WeeFIM by one [38,39,40,41,42,43]. When used to follow a large population, the cost factor of the WeeFIM may be offset by the time used to complete the assessment. The use of a validated tool to document the impact of a treatment on a child’s functional abilities may prove important as such information can be used to document a decrease in the burden and costs for caring for a child with a disability and thus justify continued use of a treatment to treat disabled children by the entity funding the treatment.
As the realization that assessment of the type and distribution of hypertonia was inadequate for candidate selection, attention was also turning towards functional assessment. In 2001 the World Health Association published a revised classification system for individuals with disabilities, the International Classification of Functioning, Disability, and Health (ICF) [44,45,46]. The premise for the revision was to describe and classify according to ability or the components of health [46]. It broadened the concept of disability from simply residing inside the individual to one that also included the ability of the individual to interact with their environment. This will and should increasingly come into play when we consider treating an individual with a disability. We will first be expected to correctly assess what a person is capable of doing. This is reflected in articles such as Schwartz’s where he seeks to analyze an individual’s ability to control the motor system when walking and not to simply document the abnormalities present [31]. In the future, we should anticipate the need to assess how successfully a person can interact with his or her environment and whether the same outcome could be obtained by either modifying the environment or giving the individual a tool to enable him or her to successfully interact as would be expected if a proposed surgical treatment were employed. This should result in much more rigorous thought being given to a patient’s goals and how best to achieve them.
Imaging
Dystonia has been a contraindication for a child to undergo a SDR. Its presence can be difficult to discern on examination, so authors have sought other means of identifying a risk for its presence. Engsberg et al. reported using MRI in screening their patients, looking for indications of damage to the basil ganglia [47]. In 2007 Cole mentioned using MRI of the brain and spinal cord but did not report on the results of this study in their patients or describe how the information obtained was used [48]. Grunt et al. described using MRIs of the brain and, in specific, T2 white matter changes that extended into the basil ganglia and thalamus as markers for risk of dystonia [49]. When present, they used it as a contraindication for treating a child with a SDR, while using MRI signs of basil ganglia and thalamic injury as indicators for the substrate responsible for dystonia in children has merit what is lacking in the validation. Undoubtedly there is a very low risk for a false negative with regard to a child having dystonia but no evidence of such injury on MRI. Would a SDR relief such a patient of his/her hypertonia? Conversely are MRI findings of T2 changes in the basal ganglia and thalmus contraindications for performing a SDR on a child, even if ther is no evidence of dystonia. The use of MRI in patient selection, while promising, requires more study.
Surgical technique
Surgical exposure
SDR, as described by Peacock, consisted of a L2-S1 laminectomy exposure of the spinal canal for the work to be done on the cauda equina. There have been many modifications to this. One of the first was to move from performing a laminectomy to performing a laminotomy for the lumbar segments and to then replace the laminar roof at the completion of the case [50]. The technique as first described by Raimondi in 1976 required drilling a trough into the lamina just medial to the facet joints and using a chisel to free the laminar roof [51]. The availability of high speed drills with cutting bits and protective foot plates allowed for a more rapid removal of the laminar roof, thus leading to a rapid adoption of their use for performing an osteoplastic laminotomy by many surgeons doing multilevel SDRs.
As early as 1990, surgeons began to explore more limited exposures of the cauda equina to perform SDRs. Lazareff et al. reported his results on 30 patients who underwent a L5-S1 laminectomy and SDR confined to the L4, L5, and S1 roots using Fasano’s selection criteria for identifying those rootlets to be sectioned. On average 40% of the L4 and 50% of the L5 and S1 sensory rootlets were sectioned in the children. He reported good relief from spasticity in the hip flexors, hip adductors, knee flexors, and plantar flexors except in 3 children who still had moderate spasticity in their plantar flexors. Barolat’s manuscript in 1991 reported on a technique of using a L1 laminectomy to expose the cauda equina to perform SDR [52]. After performing the laminectomy, the L1 nerve root was identified as it exited the dural sleeve, and the S1 nerve root was identified as being the largest of the medial nerve roots. The L2 nerve root was then identified as being just medial to L1. In his report, he stated that L3–L5 nerve roots could not be differentiated from one another as they were seen as “…a group of rootlets.” He went on to stress the importance of placing colored threads around each identified nerve’s dorsal root prior to proceeding with further dissection to secure their identity during the remainder of the procedure. In 1993 Park et al. first described his limited laminectomy to expose the cauda equina at the caudal end of the conus medullaris [53]. The technique first exposed the L1–L2 interspace and then identifying the conus’s tip using the intraoperative ultrasound. When needed, the interspace below or above was also exposed to allow a more extensive investigation with the ultrasound to identify tip of the conus. The lamina immediately rostral to the interspace containing the conus tip was then removed. Using the surgical microscope, he would then identify the L2 nerve root at the L1–2 interspace. The root was then followed back to the cord and separated into its dorsal and ventral components, and the L2 dorsal root and the caudal dorsal roots were elevated medially to allow him to place a cotton patty between them and their ventral roots. The conus was then inspected, and the S3–S5 dorsal roots were identified by their size being significantly smaller than S2. A silastic sheet was placed to separate the L2 through S2 dorsal roots from the remainder of the cauda equina. He cautioned that in some cases differentiation of S3 from S2 could be difficult and that one should be conservative in such cases, sparing the root just rostral to the presumed S3–S5 complex from lesioning. He concluded that the technique offered the advantages of providing the option to lesion the dorsal root of L1, performing the rootlet sectioning below the conus, minimizing the risk for postoperative weakness, and reducing the risk for developing postoperative spine deformities. However, he felt that the procedure was more demanding than the multi-laminotomy for performing a SDR due to the difficulty in identifying the L3-S1 dorsal nerve roots. He has subsequently published his results using this technique, and it has been adopted by many [19, 27, 48, 54,55,56,57,58,59,60,61]. In 2015 Sindou described his technique for limiting the required laminectomies to perform a SDR [62]. Prior to surgery, he constructed a surgical map of the dorsal roots to be targeted for lesioning. This map was based on the muscles containing problematic spasticity and the afferent nerve roots commonly thought to innervate them. At surgery access to targeted sensory roots was accomplished by an enlargement of the interlaminar space while leaving the spinous processes and interspinous ligament intact. In discussing his technique, he raised concern about the need to securely identify nerves being targeted during a SDR. He was concerned that the procedures advocating more limited exposures of the cauda equina at or just below the conus had the risk of misidentifying the level of the nerve roots being worked upon. He also raised a concern that there was a known variability in the motor nerve innervation patterns in the leg’s muscles and thus securing knowledge of a nerve’s spinal level was important for correctly interpreting response patterns to stimulation [63]. He favored his approach as it lessened the risk for postoperative spinal deformities and maintained the ability to securely identify each nerve root he would be potentially lesioning.
At present the surgeon has several techniques to choose from to expose the nerve roots of the cauda equina for lesioning. In deciding which best fits the surgeon’s needs, he or she should first consider what selection criteria will be used for lesioning. Will the lesioning be done using neurophysiological mapping, and if so, will this be done in the setting of scientific assessment of the mapping technique or simply as suggestive guidance for lesioning to reach a predetermined percentage of lesioning in the cauda equina? The former obviously mandates correct identification of the spinal level of the nerve, and studies such as done by Schirmer et al. clearly show the advantage of secure anatomical knowledge of the nerve root exit level and the dangers of simply using electrical mapping of evoked muscle activity to identify a nerve’s spinal level [63].
Neurophysiological mapping and lesioning
The SDR introduced in the mid-1980s used Fasano’s technique for electrically stimulating sensory nerve roots and their rootlets while monitoring the pattern of muscles responding as well as their EMG recordings [4, 64]. In his 1982 paper, Peacock et al. described a “normal root’s/rootlet’s” response as “…characterized by: (i) a single muscular contraction at 50 stimuli per second; and (ii) no diffusion of muscular contraction to muscle groups other than the one being stimulated” and an abnormal response as “(i) a tetanic muscular contraction at 50 stimuli per second; and (ii) a diffusion of muscular contraction to muscle groups other than those being stimulated.” [2]. Early on questions arose about inconsistency in response patterns obtained using Fasano’s stimulation parameters and occurrences of muscle response patterns not predicted in his manuscripts. Variability in response to repetitive stimulation was noted in a presentation I gave at the annual scientific meeting of the American Society of Pediatric Neurosurgeons in 1990 [65]. Others reported finding that Fasano’s and Peacock’s criteria of tetanic contraction of a muscle in response to a sensory root being stimulated by a threshold, 50 Hz train, could be seen in “normal” patients without physical signs of spasticity preoperatively or a clinical history of cerebral palsy [66, 67]. Other selection criteria to be used to select candidate sensory rootlets to be lesioned have been advanced. Storrs used the H1/H2 reflex recovery ratio obtained by stimulating the exposed sensory rootlets of the L2-S1 roots and included the S2 sensory rootlets when clawing of the toes was present preoperatively, labeling ratios of > 50% as abnormal [68]. They typically lesioned 2/3s of the rootlets tested finding a 80% drop in spasticity as measured by the Ashworth Scale at the 6-month postoperative examination with this reduction persisting at 7–8 years postoperatively. Bales et al. have described their technique of establishing “nontargeted” leg muscles (those without problematic spasticity) and “targeted” leg muscles (those with problematic spasticity) preoperatively and then testing the sensory nerves via a single level laminectomy at the level directly below the conus (typically L2) [58]. The sensory rootlets were then differentiated from the motor roots using electrical stimulation (threshold needed to elicit a motor contraction much lower in motor than in sensory). Sensory rootlets, when stimulated, that elicited response in “targeted muscles” were then “…graded as normal, slightly abnormal, or markedly abnormal based on the presence of one or more of the following: a persistent response; a waxing and waning response; an increasing, decreasing, or burst response, and a spread of tetanic response to other muscle groups.” “If the response is markedly abnormal (as defined by the above criteria), 75–90% of the rootlet is cut. If slightly abnormal, 50% of the rootlet is cut. If the response to tetanic stimulation is normal but the rootlet elicits a response only in affected muscle groups, 50% of the rootlet is incised. If the rootlet produces a stimulation response in a nontarget muscle group but does produce an abnormal response to tetanic stimulation, then 50% of the rootlet is cut. Normally responding rootlets in nontarget muscle groups are preserved.” They report their patients experience a mean Ashworth Scale drop of 2.08 at a mean follow-up of 15 months. Zang et al. have reported success in using the same technique to treat children with spastic hemiplegic CP [69].
We clearly need more knowledge about the pattern of muscle contraction elicited when sensory nerve roots are stimulated in normal children and those with spasticity to justify our selection criteria for lesioning of sensory rootlets to manage muscle spasticity. At best, currently used selection criteria give us a sense of which nerves when lesioned will assist in normalizing muscle tone in a child with spastic cerebral palsy, but they by no means guarantee that we will avoid residual spasticity and/or have undesired effects on the muscle tone in the legs or create undesired sensory abnormalities.
Complications
Spinal deformities
In 1990 Peter reported on the incidence of spinal abnormalities in 51 patients who were 1–7 years postoperative from undergoing SDR [70]. They found that 9 patients had scoliosis, 3 kyphosis, 4 lordosis, 2 degenerative osteophytic changes, and 5 with spondylosis with 2 having grade 1 spondylolisthesis. The scoliosis was described as mild in most cases and that it occurred in children with spastic quadriplegia more commonly. They noted that this incidence was less than that generally found in the CP population who had not undergone lumbar laminectomies. They could not explain the occurrence of spondylolysis but were concerned that it was associated with the loss of support for the posterior lumbosacral spine due to the extensive laminectomies their patients had undergone. Park, in his 1993 paper on the limited, single laminectomy technique, mentions a lessened risk for postoperative spinal deformity as a justification for his technique, and this has been mentioned widely in subsequent papers describing experience with this technique [27, 48, 52, 53, 58,59,60, 62].
Children with CP are at risk for the development of scoliosis, and this risk should be borne in mind when considering a treatment for their hypertonia. Mild scoliosis of less than 25° is generally observed while bracing can be considered for moderate scoliosis (25° to 45°). When scoliosis becomes severe (> 45°), surgical correction/stabilization is considered to avoid the pain, limitations on movement and breathing that can be seen in a child with severe scoliosis [71]. In Peter’s original report, the incidence of scoliosis was 18%; Spiegel et al. found it in 17% of his patients after surgery; Johnson et al. in 24% after SDR; in Steinbok et al.’s paper, scoliosis of greater than 35° was found in 6% of 104 patients receiving SDR; and in Golan et al.’s report, 3% had curves greater than 25° [70, 72,73,74,75]. When Langerak et al. looked at the Cape Town SDR patients who were 17+ years out from their SDRs, they found that 17 of the 30 patients had some evidence of scoliosis with only 5 experiencing pain of a mild to moderate degree which could be managed conservatively [76]. They concluded that overall, the reported incidence of scoliosis was what one expects in an unoperated population of children with CP. Persson-Bunke et al., in a population-based study from Sweden, reviewed the records of 666 children with CP where scoliosis screening was part of their regular health care [77]. Overall, 116 of 666 have some evidence of scoliosis (17%) with 45/666 (7%) having curves of > 20°. They went on to analyze the incidence as a function of GMFCS level finding that the overall incidence of scoliosis in all patients in either level 1 or 2 was 20% with none having curves exceeding 20°, while 20% of level 3 children had some evidence of scoliosis with only 2 having curves exceeding 20°, and 43% of children in levels 4 and 5 children had scoliosis with 22% exceeding 20°. This study is valuable given its population-based nature and puts the reported problem of scoliosis in the post-SDR patients into context, i.e., what is the expected risk for occurrence of moderate to severe scoliosis in the overall CP population. For each of the CP outcome studies citing postoperative incidence of scoliosis, one should first ask what is the degree (mild, moderate, or severe) of scoliosis being reported and the range of GMFCS levels for the patients being reported on before determining whether or not the incidence of scoliosis is elevated above what is expected for a child with cerebral palsy. Overall, it appears that the case for SDRs done using extensive laminectomies or laminoplasties raising the incidence of scoliosis still needs to be proved.
Progressive lordosis of the lumbosacral spine is somewhat more problematic. It is a relatively common finding in children with CP. It is typically a result of tightness in the hip flexors creating a forward tilt of the pelvis that is, at first, mobile but can become fixed in adolescence or adulthood. When the tilt of the pelvis exceeds 20°, the angulation of the sacrum nears horizontal (lordotic curve > 60–70°), and the patient begins to complain of back pain [78, 79]. Additionally, the presence of a lordotic curve exceeding 50° increases the incidence of spondylolisthesis from 21 to 29%, and this hyperlordosis is associated with a L5-S1 facet arthropathy in 67% [79]. Langerak et al. reported hyperlordosis in 6 of their 30 patients when their x-rays were reviewed 17+ years after their SDR [76]. Johnson et al. found the mean lordotic curvature in the 34 patients they analyzed to progress from a mean of 17° preoperatively to 54° with 17 having lordotic curves > 60° at a mean follow-up of 8.6 years [74]. Steinbok et al. found lordotic curvatures of > 54° in 10/47 children who had x-rays 1+ year (1–13.6-year range) after their SDR [72]. An important point is made by Spiegel et al. in their paper that assessment of the degree of lordotic curvature present is susceptible to the position of the patient for the films, citing the preoperative lordotic curve in their patients being 17° when sitting but 45° when standing [73]. The magnitude of this difference persisted in their postoperative measurements (18° when sitting and 45° when standing). Many reports do not document the patients’ positioning when the x-rays are taken making their interpretation difficult. Additionally, Lee et al. found a significant inter-observer variability (0.59, 0.315–0.770 CI) in interpreting plain lateral x-rays of the lateral spine when evaluating 184 children for lordosis [80]. The concerns raised by Spiegel et al. and Lee et al. should be kept in mind when reviewing reports of the incidence of lordosis in children who have undergone SDR.
Spondylolysis and spondylolisthesis can develop after a child undergoes a SDR. The occurrence of spondylolysis and spondylolisthesis in children with cerebral palsy is important because of the associated pain and instability. Spondylolysis may be seen in 4% of children with CP by 6 years of age due to repetitive hyperextension causing stress fractures in the pars interarticularis of the lumbar spine [81]. This incidence increases to 6% by age 14 years. Its presence is thought to be a risk factor for the development of spondylolisthesis. Peter first reported on the risk for spondylolysis and spondylolisthesis in 1990, finding that 5 of his 51 patients had evidence of spondylolysis with two having a grade 1 spondylolisthesis [70]. At long-term follow-up, his group reported that 11 of 30 had developed spondylolysis, while only one of these had spondylolisthesis [76]. Spiegel found spondylolisthesis in 9 of 51 diplegics 2+ years after they had undergone SDR [73]. Eight of 34 children reviewed by Johnson et al. 5+ years after undergoing SDR had spondylolisthesis with two having had it before their surgery [74]. Golan, reporting on the postoperative x-rays of 87 children taken 1+ years after undergoing SDR, found 6/87 with grade 1 spondylolisthesis and 12/87 with grade 2 spondylolisthesis at L5-S1 [75]. They also found spondylolysis in 11 of 87 patients’ follow-up x-rays. All of these outcome studies reported rates of spondylolysis and spondylolisthesis higher than would be expected in the general CP population, so this risk should be taken into consideration when following children who have undergone SDR. It has been recommended that physical therapy measures targeting anterior tilting of the pelvis due to hip flexor tightness can be helpful in countering the risk for developing spondylolysis and spondylolisthesis [81].
Spinal stenosis is a rare condition for children with cerebral palsy and has not been widely reported to occur after SDR. Gooch et al. reported on 2 cases of patients developing pain and numbness in legs several years after undergoing a SDR with replacement of the laminar roof [82]. Their MRIs showed lumbar canal narrowing consistent with lumbar stenosis. Both had symptomatic resolution with surgical decompression of the lumbar spinal canal leading the authors to conclude that their patients had developed symptomatic lumbar stenosis as a complication of their SDR. Langerak et al. also reported on the development of spinal stenosis, finding it in 9 of 30 patients they reviewed 17+ years after their SDR with only one reporting difficulties with pain [76]. All their patients had laminectomies over multiple levels of the lumbar spine as part of their SDR procedure. Given the limited number of reports of spinal stenosis and the varying techniques of exposure used, it is difficult to assign the degree of risk for its occurrence in children undergoing SDR or identifying particular risk factors for its occurrence. It does bear watch as more long-term outcome reports become available.
Low-back and radicular pain
Pain is a potential complication for spine surgery, and it has been reported as occurring in children long after they underwent SDRs. Park et al. surveyed 95 of their patients who were 20–28 years after undergoing SDR [83]. 25/95 were experiencing back and/or leg pain with the mean intensity being 4.2/10 on the numeric rating scale (NRS), a 0–10 scale with zero being no pain, and 10 the worst pain imaginable [83, 84]. Nine of their patients described the pain as being constant. Hurvitz et al. surveyed 99 patients 18–22 years after they had undergone SDR using multilevel laminectomies [85]. “About” half reported chronic back pain with 44% experiencing pain during the week prior to being surveyed. The reported pain had an intensity of 5 on the NRS.
Others have reported on the incidence of pain in long-term outcome reviews of their patients. Johnson et al. reported 10 of 34 patients complaining of low back pain [74]. Langerak et al. surveyed 30 patients 10 or more years after undergoing SDR in Cape Town using the Oswestry Low Back Pain Disability Questionnaire (QDI) [76, 86]. The QDI divides scoring into 5 levels of disabling pain: minimally disabling pain—no treatment needed; moderately disabling pain—pain when sitting, lifting, and standing but not affecting level of daily activities; severe disabling pain—pain adversely affecting ability to engage in daily activities; crippling pain—all aspects of life adversely affected and surgical intervention required; and a last group where 80%+ of response to questionnaire are positive for pain due to patient being bed-bound or to patient exaggeration of impact of pain on daily life. Langerak et al. found that 23/30 of their patients scored as being minimally disabled by back or leg pain and 7 as having moderately disabling pain [76]. Low back pain occurred weekly in 2 patients and daily in 5. It was reported in 5 of 17 with scoliosis, one of two with kyphosis, 4 of 12 with hyperlordosis, 3 of 11 with spondylolysis, and in the single patient with spondylolisthesis. Eight of 20 patients reporting pain also reported using medications intermittently for their pain. These long-term follow-up surveys identify pain in a high percentage of patient’s questioned. The pain, in most cases, seemed not to interfere with daily activities and, when needed, was managed medically.
Conclusions
Since its popularization in the 1990s, SDR has dramatically matured and is now a widely accepted tool for managing hypertonia in children with CP. It appears that it may also useful for managing hypertonia of other etiology. We now have validated tools for assessing children for the procedure and for following them afterwards. We have a variety of surgical techniques to choose from for performing the procedure and information about long-term complications associated with the procedure that can be used to both help in selecting how to perform it and to better inform patients and families who are considering undergoing a SDR. When thoughtfully applied, this knowledge can continue to provide valuable understanding of the physiology of the nervous system, providing benefit not only to patients undergoing the procedure but also to science in general.
References
Abbott R, Forem SL, Johann M (1989) Selective posterior rhizotomy for the treatment of spasticity: a review. Childs Nerv Syst 5:337–346
Peacock WJ, Arens LJ (1982) Selective posterior rhizotomy for the relief of spasticity in cerebral palsy. S Afr Med J 62:119–124
Peacock WJ, Eastman RW (1981) The neurosurgical management of spasticity. S Afr Med J 60:849–850
Fasano VA, Broggi G, Barolat-Romana G, Sguazzi A (1978) Surgical treatment of spasticity in cerebral palsy. Childs Brain 4:289–305
Peacock WJ, Arens LJ, Berman B (1987) Cerebral palsy spasticity. Selective posterior rhizotomy. Pediatr Neurosci 13:61–66
Lance J, Young R, LKoella W (1980) Symposium synopsis. In: Feldman R (ed) Spasticity: disordered motor control. Year Book Medical Publishers, Chicago
Loewen P, Steinbok P, Holsti L, MacKay M (1998) Upper extremity performance and self-care skill changes in children with spastic cerebral palsy following selective posterior rhizotomy. Pediatr Neurosurg 29:191–198
Albright AL, Barry MJ, Fasick MP, Janosky J (1995) Effects of continuous intrathecal baclofen infusion and selective posterior rhizotomy on upper extremity spasticity. Pediatr Neurosurg 23:82–85
Kinghorn J (1992) Upper extremity functional changes following selective posterior rhizotomy in children with cerebral palsy. Am J Occup Ther 46:502–507
Lazareff JA, Mata-Acosta AM, Garcia-Mendez MA (1990) Limited selective posterior rhizotomy for the treatment of spasticity secondary to infantile cerebral palsy: a preliminary report. Neurosurgery 27:535–538
McLaughlin JF, Bjornson KF, Astley SJ, Hays RM, Hoffinger SA, Armantrout EA, Roberts TS (1994) The role of selective dorsal rhizotomy in cerebral palsy: critical evaluation of a prospective clinical series. Dev Med Child Neurol 36:755–769
Morota N, Kameyama S, Masuda M, Oishi M, Aguni A, Uehara T, Nagamine K (2003) Functional posterior rhizotomy for severely disabled children with mixed type cerebral palsy. Acta Neurochir Suppl 87:99–102
Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39:214–223
Ailon T, Beauchamp R, Miller S, Mortenson P, Kerr JM, Hengel AR, Steinbok P (2015) Long-term outcome after selective dorsal rhizotomy in children with spastic cerebral palsy. Childs Nerv Syst 31:415–423
Ingale H, Ughratdar I, Muquit S, Moussa AA, Vloeberghs MH (2016) Selective dorsal rhizotomy as an alternative to intrathecal baclofen pump replacement in GMFCS grades 4 and 5 children. Childs Nerv Syst 32:321–325
Buizer AI, van Schie PEM, Bolster EAM, van Ouwerkerk WJ, Strijers RL, van de Pol LA, Stadhouder A, Becher JG, Vermeulen RJ (2017) Effect of selective dorsal rhizotomy on daily care and comfort in non-walking children and adolescents with severe spasticity. Eur J Paediatr Neurol 21:350–357
Gump WC, Mutchnick IS, Moriarty TM (2013) Selective dorsal rhizotomy for spasticity not associated with cerebral palsy: reconsideration of surgical inclusion criteria. Neurosurg Focus 35:E6
Reynolds RM, Morton RP, Walker ML, Massagli TL, Browd SR (2014) Role of dorsal rhizotomy in spinal cord injury-induced spasticity. J Neurosurg Pediatr 14:266–270
Kai M, Yongjie L, Ping Z (2014) Long-term results of selective dorsal rhizotomy for hereditary spastic paraparesis. J Clin Neurosci 21:116–120
Elk B (1980) Preoperative assessment and postoperative surgical occupational therapy for children who have undergone a selective posterior rhizotomy. S Afr J Occup Ther 14:49–50
Abou Al-Shaar H, Imtiaz MT, Alhalabi H, Alsubaie SM, Sabbagh AJ (2017) Selective dorsal rhizotomy: a multidisciplinary approach to treating spastic diplegia. Asian J Neurosurg 12:454–465
Oudenhoven LM, van der Krogt MM, Romei M, van Schie PEM, van de Pol LA, van Ouwerkerk WJR, Harlaar J, Buizer AI (2019) Factors associated with long-term improvement of gait after selective dorsal rhizotomy. Arch Phys Med Rehabil 100:474–480
Steinbok P (2007) Selective dorsal rhizotomy for spastic cerebral palsy: a review. Childs Nerv Syst 23:981–990
Roberts A, Stewart C, Freeman R (2015) Gait analysis to guide a selective dorsal rhizotomy program. Gait Posture 42:16–22
Wong AM, Chen CL, Hong WH, Tang FT, Lui TN, Chou SW (2000) Motor control assessment for rhizotomy in cerebral palsy. Am J Phys Med Rehabil 79:441–450
Kim HS, Steinbok P, Wickenheiser D (2006) Predictors of poor outcome after selective dorsal rhizotomy in treatment of spastic cerebral palsy. Childs Nerv Syst 22:60–66
D'Aquino D, Moussa AA, Ammar A, Ingale H, Vloeberghs M (2018) Selective dorsal rhizotomy for the treatment of severe spastic cerebral palsy: efficacy and therapeutic durability in GMFCS grade IV and V children. Acta Neurochir 160:811–821
Graham D, Aquilina K, Mankad K, Wimalasundera N (2018) Selective dorsal rhizotomy: current state of practice and the role of imaging. Quant Imaging Med Surg 8:209–218
Munger ME, Aldahondo N, Krach LE, Novacheck TF, Schwartz MH (2017) Long-term outcomes after selective dorsal rhizotomy: a retrospective matched cohort study. Dev Med Child Neurol 59:1196–1203
Wang KK, Munger ME, Chen BPJ, Novacheck TF (2018) Selective dorsal rhizotomy in ambulant children with cerebral palsy: an observational cohort study. J Child Orthop 12:413–427
Schwartz MH, Rozumalski A, Steele KM (2016) Dynamic motor control is associated with treatment outcomes for children with cerebral palsy. Dev Med Child Neurol 58:1139–1145
Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S (1989) The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol 31:341–352
Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ (2000) Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther 80:873–885
Theologis T, Wright J (2015) Is 3-D gait analysis essential? By Professor James Wright: Introduction by Mr. Tim Theologis. Gait Posture 42:227–229
Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F (2006) Motor patterns in human walking and running. J Neurophysiol 95:3426–3437
Haley SMCW, Ludlow LH et al (1992) Pediatric evaluation of disability inventory: development, standardization and administration manual. Trustees of Boston University, Boston
Msall ME, DiGaudio K, Rogers BT, LaForest S, Catanzaro NL, Campbell J, Wilczenski F, Duffy LC (1994) The functional independence measure for children (WeeFIM). Conceptual basis and pilot use in children with developmental disabilities. Clin Pediatr (Phila) 33:421–430
Josenby AL, Wagner P, Jarnlo GB, Westbom L, Nordmark E (2015) Functional performance in self-care and mobility after selective dorsal rhizotomy: a 10-year practice-based follow-up study. Dev Med Child Neurol 57:286–293
Mittal S, Farmer JP, Al-Atassi B et al (2002) Functional performance following selective posterior rhizotomy: long-term results determined using a validated evaluative measure. J Neurosurg 97:510–518
Nordmark E, Jarnlo GB, Hagglund G (2000) Comparison of the gross motor function measure and paediatric evaluation of disability inventory in assessing motor function in children undergoing selective dorsal rhizotomy. Dev Med Child Neurol 42:245–252
Nordmark E, Josenby AL, Lagergren J, Andersson G, Stromblad LG, Westbom L (2008) Long-term outcomes five years after selective dorsal rhizotomy. BMC Pediatr 8:54
Steinbok P, Tidemann AJ, Miller S, Mortenson P, Bowen-Roberts T (2009) Electrophysiologically guided versus non-electrophysiologically guided selective dorsal rhizotomy for spastic cerebral palsy: a comparison of outcomes. Childs Nerv Syst 25:1091–1096
van Schie PE, Vermeulen RJ, van Ouwerkerk WJ, Kwakkel G, Becher JG (2005) Selective dorsal rhizotomy in cerebral palsy to improve functional abilities: evaluation of criteria for selection. Childs Nerv Syst 21:451–457
International Classification of Functioning, Disability and Health (ICF) (2001) (Accessed August 12, 2019, at https://www.who.int/classifications/icf/en/)
Towards a Common Language for Functioning, Disability and Health. ICF. World Health Organization (2002) (Accessed August 12, 2019, at https://www.who.int/classifications/icf/icfbeginnersguide.pdf?ua=1)
Rosenbaum P, Stewart D (2004) The World Health Organization international classification of functioning, disability, and health: a model to guide clinical thinking, practice and research in the field of cerebral palsy. Semin Pediatr Neurol 11:5–10
Engsberg JR, Ross SA, Park TS (1999) Changes in ankle spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. J Neurosurg 91:727–732
Cole GF, Farmer SE, Roberts A, Stewart C, Patrick JH (2007) Selective dorsal rhizotomy for children with cerebral palsy: the Oswestry experience. Arch Dis Child 92:781–785
Grunt S, Fieggen AG, Vermeulen RJ, Becher JG, Langerak NG (2014) Selection criteria for selective dorsal rhizotomy in children with spastic cerebral palsy: a systematic review of the literature. Dev Med Child Neurol 56:302–312
Abbott R, Feldstein N, Wisoff J, Epstein F (1992) Osteoplastic laminotomy in children. Pediatr Neurosurg 18:153–156
Raimondi AJ, Gutierrez FA, Di Rocco C (1976) Laminotomy and total reconstruction of the posterior spinal arch for spinal canal surgery in childhood. J Neurosurg 45:555–560
Barolat G (1991) Dorsal selective rhizotomy through a limited exposure of the cauda equina at L-1. Technical note. J Neurosurg 75:804–807
Park TS, Gaffney PE, Kaufman BA, Molleston MC (1993) Selective lumbosacral dorsal rhizotomy immediately caudal to the conus medullaris for cerebral palsy spasticity. Neurosurgery 33:929–933 discussion 33-4
Engsberg JR, Olree KS, Ross SA, Park TS (1998) Spasticity and strength changes as a function of selective dorsal rhizotomy. J Neurosurg 88:1020–1026
Engsberg JR, Ross SA, Olree KS, Park TS (2000) Ankle spasticity and strength in children with spastic diplegic cerebral palsy. Dev Med Child Neurol 42:42–47
Engsberg JR, Ross SA, Wagner JM, Park TS (2002) Changes in hip spasticity and strength following selective dorsal rhizotomy and physical therapy for spastic cerebral palsy. Dev Med Child Neurol 44:220–226
Engsberg JR, Ross SA, Collins DR, Park TS (2006) Effect of selective dorsal rhizotomy in the treatment of children with cerebral palsy. J Neurosurg 105:8–15
Bales J, Apkon S, Osorio M, Kinney G, Robison RA, Hooper E, Browd S (2016) Infra-conus single-level laminectomy for selective dorsal rhizotomy: technical advance. Pediatr Neurosurg 51:284–291
Funk JF, Haberl H (2016) Monosegmental laminoplasty for selective dorsal rhizotomy--operative technique and influence on the development of scoliosis in ambulatory children with cerebral palsy. Childs Nerv Syst 32:819–825
Graham D, Aquilina K, Cawker S, Paget S, Wimalasundera N (2016) Single-level selective dorsal rhizotomy for spastic cerebral palsy. J Spine Surg 2:195–201
Summers J, Coker B, Eddy S, Elstad M, Bunce C, Bourmpaki E, Pennington M, Aquilina K, Cawker S, Edwards R, Goodden J, Hawes S, McCune K, Pettorini B, Smith J, Sneade C, Vloeberghs M, Patrick H, Powell H, Verity C, Peacock JL, Selective Dorsal Rhizotomy Steering Committee (2019) Selective dorsal rhizotomy in ambulant children with cerebral palsy: an observational cohort study. Lancet Child Adolesc Health 3:455–462
Sindou M, Georgoulis G (2015) Keyhole interlaminar dorsal rhizotomy for spastic diplegia in cerebral palsy. Acta Neurochir 157:1187–1196
Schirmer CM, Shils JL, Arle JE, Cosgrove GR, Dempsey PK, Tarlov E, Kim S, Martin CJ, Feltz C, Moul M, Magge S (2011) Heuristic map of myotomal innervation in humans using direct intraoperative nerve root stimulation. J Neurosurg Spine 15:64–70
Fasano VA, Barolat-Romana G, Zeme S, Squazzi A (1979) Electrophysiological assessment of spinal circuits in spasticity by direct dorsal root stimulation. Neurosurgery 4:146–151
Abbott R, Deletes V, Spielholz N, Wisoff J, Epstein F (1990) Selective posterior rhizotomy: pitfalls in monitoring. Concepts in Pediatric Neurosurgery 10:187–195
Cohen AR, Webster HC (1991) How selective is selective posterior rhizotomy? Surg Neurol 35:267–272
Steinbok P, Langill L, Cochrane DD, Keyes R (1992) Observations on electrical stimulation of lumbosacral nerve roots in children with and without lower limb spasticity. Childs Nerv Syst 8:376–382
Storrs BB, Nishida T (1988) Use of the 'H' reflex recovery curve in selective posterior rhizotomy. Pediatr Neurosci 14:120–123
Zhan Q, Tang L, Wang Y et al (2019) Feasibility and effectiveness of a newly modified protocol-guided selective dorsal rhizotomy via single-level approach to treat spastic hemiplegia in pediatric cases with cerebral palsy. Childs Nerv Syst
Peter JC, Hoffman EB, Arens LJ, Peacock WJ (1990) Incidence of spinal deformity in children after multiple level laminectomy for selective posterior rhizotomy. Childs Nerv Syst 6:30–32
Sheehan DD, Grayhack J (2017) Pediatric scoliosis and kyphosis: an overview of diagnosis, management, and surgical treatment. Pediatr Ann 46:e472–ee80
Steinbok P, Hicdonmez T, Sawatzky B, Beauchamp R, Wickenheiser D (2005) Spinal deformities after selective dorsal rhizotomy for spastic cerebral palsy. J Neurosurg 102:363–373
Spiegel DA, Loder RT, Alley KA, Rowley S, Gutknecht S, Smith-Wright DL, Dunn ME (2004) Spinal deformity following selective dorsal rhizotomy. J Pediatr Orthop 24:30–36
Johnson MB, Goldstein L, Thomas SS, Piatt J, Aiona M, Sussman M (2004) Spinal deformity after selective dorsal rhizotomy in ambulatory patients with cerebral palsy. J Pediatr Orthop 24:529–536
Golan JD, Hall JA, O'Gorman G et al (2007) Spinal deformities following selective dorsal rhizotomy. J Neurosurg 106:441–449
Langerak NG, Vaughan CL, Hoffman EB, Figaji AA, Fieggen AG, Peter JC (2009) Incidence of spinal abnormalities in patients with spastic diplegia 17 to 26 years after selective dorsal rhizotomy. Childs Nerv Syst 25:1593–1603
Persson-Bunke M, Hagglund G, Lauge-Pedersen H, Wagner P, Westbom L (2012) Scoliosis in a total population of children with cerebral palsy. Spine (Phila Pa 1976) 37:E708–E713
Bleck EE (1987) In: Bleck EE (ed) Orthopeaedic management in cerebral palsy, 1st edn. Mac Keith Press, London, p 138
Morrell DS, Pearson JM, Sauser DD (2002) Progressive bone and joint abnormalities of the spine and lower extremities in cerebral palsy. Radiographics 22:257–268
Lee SY, Chung CY, Lee KM, Kwon SS, Cho KJ, Park MS (2016) Annual changes in radiographic indices of the spine in cerebral palsy patients. Eur Spine J 25:679–686
Murphy KP (2009) Cerebral palsy lifetime care - four musculoskeletal conditions. Dev Med Child Neurol 51(Suppl 4):30–37
Gooch JL, Walker ML (1996) Spinal stenosis after total lumbar laminectomy for selective dorsal rhizotomy. Pediatr Neurosurg 25:28–30
Park TS, Liu JL, Edwards C, Walter DM, Dobbs MB (2017) Functional outcomes of childhood selective dorsal rhizotomy 20 to 28 years later. Cureus 9:e1256
Edelen MO, Saliba D (2010) Correspondence of verbal descriptor and numeric rating scales for pain intensity: an item response theory calibration. J Gerontol A Biol Sci Med Sci 65:778–785
Hurvitz EA, Marciniak CM, Daunter AK, Haapala HJ, Stibb SM, McCormick SF, Muraszko KM, Gaebler-Spira D (2013) Functional outcomes of childhood dorsal rhizotomy in adults and adolescents with cerebral palsy. J Neurosurg Pediatr 11:380–388
Fairbank J, Couper J, Davies J, O'Brien J (1980) The Oswestry low back pain disability questionnaire. Physiotherapy 66:271–273
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbott, R. The selective dorsal rhizotomy technique for spasticity in 2020: a review. Childs Nerv Syst 36, 1895–1905 (2020). https://doi.org/10.1007/s00381-020-04765-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04765-6