Abstract
Purpose
Intracranial hypertension (ICH) is a common and treatable complication after severe traumatic brain injury (sTBI) in children. Describing the incidence and risk factors for developing ICH after sTBI could impact clinical practice.
Methods
Retrospective cohort study from 2006 to 2015 at two university-affiliated level I pediatric trauma centers of children admitted with accidental or abusive TBI, a post-resuscitation Glasgow Coma Score (GCS) of 8 or less, and an invasive intracranial pressure (ICP) monitor. Bivariate and multivariable logistic regression analysis were performed to identify demographic, injury, and imaging characteristics in patients who received ICP directed therapies for ICH (ICP > 20 mmHg).
Results
Eight to 5% (271/321) of monitored patients received ICP directed therapy for ICH during their PICU stay. Ninety-seven percent of patients had an abnormality on CT scan by either the Marshall or the Rotterdam score. Of the analyzed clinical and radiologic variables, only presence of hypoxia prior to PICU arrival, female sex, and a higher Injury Severity Score (ISS) were associated with increased risk of ICH (p < 0.05).
Conclusions
In this retrospective study of clinical practice of ICP monitoring in children after sTBI, the vast majority of children had an abnormal CT scan and experienced ICH requiring clinical intervention. Commonly measured clinical variables and radiologic classification scores did not significantly add to the prediction for developing of ICH and further efforts are needed to define low-risk populations that would not develop ICH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Severe TBI is a leading cause of death and disability among children with an estimated 40–60% of survivors experiencing long-term neurologic impairments [1, 2]. In an effort to optimize care and reduce the long-term sequalae from secondary brain insults, Pediatric Critical Care Medicine (PCCM) and the Brain Trauma Foundation (BTF) endorsed pediatric guidelines for the management of sTBI in infants, children, and adolescents, first published in 2003 and updated in 2012 and 2019 [3,4,5]. Although evidence for a level 1 recommendation for ICP monitoring in children after sTBI does not exist, invasive ICP monitoring remains a current practice in the management of children with sTBI. Invasive ICP monitoring allows objective and graded use of therapies such as hyperosmolar therapies, sedatives, paralytics, barbiturates, and neurosurgical procedures to optimize brain perfusion and limit the deleterious effects of raised ICP and low cerebral perfusion pressure that may otherwise go undetected.
In clinical practice, TBI severity is classified by evaluating the level of verbal, motor, and eye responsiveness measured by the GCS and the presence of neuro-anatomical injury on CT scan. Although these two sources of information are commonly used to guide interventions, including the decision to invasively monitor ICP, studies report wide variations in ICP monitoring rates and interhospital variability in children classified as having a sTBI [6, 7]. Taken together, these findings suggest, at least in part, that the determination of children with sTBI who would develop ICH and who would necessitate interventions to lower ICP remains uncertain. Previous study literatures attempting to investigate the risk factors for ICH have been limited by small sample size, inclusion of subdural and epidural pressure monitors, and evaluating of ICP elevation only in the first 24 h after placement [8,9,10,11]. Since placing an ICP monitor carries some risk and has the potential to impact therapeutic interventions and possibly improve patient outcome, investigating factors that could help distinguish which patients are at risk for developing ICH are needed.
Our study sought to improve knowledge gaps with respect to ICP monitoring after pediatric sTBI by addressing two important topics. First, we sought to determine if certain clinical or radiological characteristics would distinguish children at risk for developing ICH after ICP monitor placement. Second, we wished to report the percentage of children that received ICP directed therapies for a value > 20 mmHg during the duration of ICP monitoring in the era after publication of the PCCM/BTF guidelines.
Materials and methods
Study design
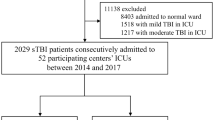
We conducted a retrospective study at two university-affiliated tertiary care level I pediatric trauma centers of consecutively admitted children to the pediatric intensive care unit (PICU) from January 2006 to December 2015. The Institutional Review Boards at Washington University in St. Louis, Missouri, and UT Southwestern Medical Center in Dallas, Texas, approved all study procedures with a waiver of written informed consent. Inclusion criteria were the following: ages 0–18 years, post-resuscitation GCS of 8 or less, accidental TBI or abusive head trauma (AHT), and an invasive ICP monitor. Diagnosis of AHT was made by the medical and child abuse teams with findings of unexplained trauma, subdural hemorrhage, and retinal hemorrhage.
ICP monitoring and patient management
All patients underwent ICP monitoring via intraparenchymal monitor (Camino ®, Integra™ LifeSciences, Plainsboro, NJ), extra ventricular drain (EVD) catheter (Codman ® BACTISEAL®, Codman & Shurtleff, Inc., Raynham, MA), or both selected at the discretion of the attending neurosurgeon. Initiation of ICP monitoring and treatment of ICH was generally guided by the 2003 and 2012 Pediatric Critical Care Medicine (PCCM) and Brain Trauma Foundation (BTF) guidelines recommending treatment for an ICP > 20 mmHg [3, 4]. All children received invasive mechanical ventilation and sedation with narcotics and/or benzodiazepines as needed for maintenance of mechanical ventilation.
Data collection
De-identified patient data were obtained from the Trauma Registry Database or electronic medical record. By thorough evaluation of each patient’s medical record, patients were separated into an ICH group (patients who received ICP directed therapy for an ICH episode during the monitoring period) and a no-ICH group (patients with no episodes of ICH that received ICP directed therapies). Importantly, patients had to have an ICH episode and have received ICP directed therapy for that episode to be included in the ICH group. Directed ICP therapies included administration of mannitol or hypertonic saline, analgesics, paralytics and/or sedatives (including pentobarbital), and decompressive craniectomy, and in cases of severe ICH with signs of herniation, transient hyperventilation. Anatomical brain injury was scored based on the admission brain CT by using the Marshall CT and Rotterdam classification [12, 13]. An experienced pediatric neurosurgeon (JL) and radiologist (MRP) assigned classification scores to all the cases while blinded to patient characteristics and ICH treatment decisions. Kappa statistical analysis revealed good agreement between the two reader’s scores for each of the classification methods. Patient outcomes were measured by PICU discharge GCS and hospital discharge 5-point Glasgow Outcome Scale (GOS) [14].
Data analysis
Independent variables associated with the presence or absence of ICH included emergency department (ED) post-resuscitation GCS, age at injury, sex, Injury Severity Score (ISS), and Rotterdam and Marshall CT score as well as pre-PICU cardiopulmonary resuscitation (CPR), hypoxia (oxygen saturation < 90%), hypotension (systolic blood pressure < 5th percentile for age), and acidosis (pH < 7.35) [15]. The unadjusted comparisons of study center and ICH status with variables and outcome were assessed using Student’s t test (normal distribution) and Man-Whitney test (non-normal distribution) and Fisher’s exact test for nominal variables and Wilcoxon rank sum test for measured and ordinal scale variables. Candidate variables for logistic regression were investigated using a stepwise selection process, with probability of entry and removal set to a p = 0.2 [16, 17]. Linearity between continuous independent variables and the log odds of ICH status was assessed graphically. To address non-linearity of predictions, post-resuscitation GCS was modeled as a > 5 versus ≤ 5. Age was modeled as both a continuous variable and an ordinal variable of groups < 5 years old, 5–10 years old, and 11–17 years old [18]. The odds ratio (OR) and receiver operator characteristic (ROC) curves/c-statistics were calculated. Finally, we took 200 bootstrap samples of the dataset, ran through the modeling process in each bootstrap sample, and compared with the initial model to calculate a measure of optimism of the original model’s c-statistic [19]. For our data analysis, statistical significance was set for an alpha level of less than 0.05. All analyses were conducted using SAS software v9.4 (Cary, NC).
Results
Incidence and predictors of patients with intracranial hypertension
A total of 321 patients met inclusion criteria from the two centers; of these (271/321), 85% developed at least one episode of ICP > 20 mmHg during their PICU stay that received ICP directed therapy. In the cohort of patients with AHT (43/48), 93.7% received ICP-directed therapies. There were no differences in median age or age ranges, mechanism of injury, ICP monitoring method or CPR, acidosis, or hypotension prior to PICU arrival between the ICH group and the no-ICH group (Table 1). The ICH group compared to the no-ICH group had a higher percentage of female patients (38.4% versus 22%; p = 0.04), higher ISS score (median [IQR]; 28 [22, 38] versus 26 [19, 20]; p < 0.01), and increased incidence of hypoxia prior to PICU arrival (31% versus 7%; p = 0.02). The post-resuscitation GCS was lower in the ICH group (median [IQR]; 3 [3, 5] versus 5 [3, 6]) but was not statistically significant.
There were no differences in sex, mechanism of injury, hypoxia or hypotension prior to PICU arrival, ICP monitoring method, hospital discharge GOS, or Marshall CT and Rotterdam score between the study centers. The percentage of patients who developed ICH was also similar between centers, Dallas (136/168) 80.1% and St. Louis (135/153) 88.2%. Patients from Dallas were younger, had lower post-resuscitation GCS score, and had increased incidence of acidosis on their initial blood gas (p < 0.01) (Table 2). While overall mortality was higher in the ICH group (23.3% versus 4%), the difference was not statistically significant. Patients in the ICH group had a worse PICU discharge GCS (median [IQR]; 11 [7, 14] versus 14 [11, 15]) and hospital discharge GOS scores (median [IQR]; 3 [2, 4] versus 3 [3, 4]) although the median value was the same in both groups (Table 3). In this cohort, there were no ICP monitor placement–related infections or complications that required neurosurgical intervention.
CT classification
In the vast majority 312/321 (97%) of ICP-monitored patients, abnormalities were noted by Marshall or Rotterdam scoring classification on admission brain CT scan. A Marshall score of 2 was the most frequently observed score in both the ICH and no-ICH groups (118/217) 43.5% and (21/50) 42% respectively and a Rotterdam score of 3 was the most common in the ICH and no-ICH groups, (90/271) 33.2% and (16/50) 32% respectively (Table 4). The Fisher’s exact test for categorical variables showed no difference in the Marshall or Rotterdam CT category classifications scores between those who developed ICH and those that did not.
Multivariable predictive models
We next performed an exploratory analysis using available clinical variables to generate a predictive model to distinguish the cohort of ICP monitored patients at risk for developing ICH. In the multivariate model, a stepwise selection process yielded a final logistic regression model that included hypoxia before arrival to the PICU, ISS, and sex. In this model, the presence of hypoxia (OR = 2.6, 95% CI 1.12–6.12; p = 0.03) and higher ISS (OR = 1.04, 95% CI 1.01–1.08; p = 0.01) increased the odds of developing ICH while male sex showed a reduction (OR = 0.46, 95% CI 0.22–0.95; p = 0.04). The ROC area under the curve for the model was 0.69 (95% CI, 0.61–0.76). Using our retrospective cohort as test data, we then calculated predicted probabilities for developing ICH based on the logistic regression model. The predictive values, sensitivity, and specificity for the model using the Youden index as the cut point for predicting ICH are summarized in Tables 5 and 6. The Youden index enables selection of an optimal cutoff point in the ROC, maximizing sensitivity and specificity [21, 22]. The internal validation using bootstrap analysis of the dataset yielded an optimism measure of 0.07 for the c-statistic and an optimism-adjusted c-statistic of 0.62 (original c-statistic = 0.69).
Discussion
Incidence and risk factors for developing intracranial hypertension
Several indirect lines of evidence support the rationale for invasive ICP monitoring in children with sTBI including a high incidence of ICH after sTBI in children, an association with ICH and worse neurologic outcome, and improved outcomes with ICP management protocols and successful ICP-lowering therapies for patients with TBI [4, 23,24,25,26,27]. However, the potential benefit of ICP monitoring remains controversial. Given the absence of level 1 or level II evidence, the current BTF pediatric guidelines specify a level III recommendation for ICP monitoring. This uncertainty may in part explain the variability of placing ICP monitors after sTBI. In children with severe head injury, ICP monitoring rates range from 32 to 59% with considerable interhospital variation ranging from 6 to 100% [11, 28]. We found a high incidence, roughly 85% of children who underwent ICP monitoring received therapy to treat ICH during the PICU which was slightly higher than those reported by Bailey et al. who found an incidence of ICH of 78% in the first 24 h of monitoring [9].
Ultimately, decisions for ICP monitor placement are based on repeated neurologic exams, imaging, neurosurgeon/hospital practice, and other information not captured by common data elements. We did not find any associations with mechanism of injury, GCS, age, or hypotension or CPR prior to PICU arrival in patients who developed ICH. Our findings are in agreement with other studies to report that GCS, mechanism of injury, and age were not predictive of elevated ICP in monitored patients [9, 10]. Forsyth et al. reported univariate associations with GCS, pupil reactivity, age, and admission CT findings with developing ICH but 44% of ICP monitors were extradural or subdural locations which are less reliable for ICP monitoring [8]. Our findings that hypoxia prior to PICU arrival, female sex, and higher ISS were significantly associated with higher rates of developing ICH emphasizes the importance of preventing hypoxia after sTBI and suggests extracranial factors may also play a role in developing ICH. Further prospective studies combining standard and novel variables along with pediatric specific radiological findings and a larger number of patients without ICH are needed to further validate our findings.
CT scan classification association with intracranial hypertension
Ninety-seven percent of patients had abnormalities on CT scan by Marshall or Rotterdam criteria. In patients with a “normal” CT scan, it is estimated that about 20% of those patients may develop ICH [8]. In our study, there were 9 patients with a Marshall score of I (no abnormalities), 5 developed ICH, and 4 did not. Consistent with other studies, the contribution of the current CT classification methods with the Rotterdam or the Marshall score in predicting development of ICH is modest at best [10]. Because neither scoring system was conceived based on pediatric TBI injury types and was designed more to prognosticate outcome rather than to predict ICH, it was not entirely unexpected that these classification systems did not contribute to improved identification of patients at risk of ICH [12, 13]. Nevertheless, our results suggest that the degree of injury based on currently available CT classifications may be less useful in determining which patients will go on to develop ICH. A future area of investigation may be to identify those patients with normal CT scans or pediatric specific CT injury classifications that may be used to predict risk for ICH.
Predictive model for developing intracranial hypertension
Improving the ability to identify patients at risk for ICH would be useful to clinicians evaluating the risk-benefit ratio of ICP monitoring. Accordingly, we attempted to identify injury or patient characteristics that could eventually undergo prospective validation to develop a predictive model that may help identify patients with sTBI who are at higher risk of ICH. While three variables were reliably associated with increased risk of ICH—sex, hypoxia prior to PICU arrival, and the ISS—the model yielded only modestly better prediction compared to the results based on the study population. In this model, a probability of 82% (maximum Youden index) would identify patients who go on to develop ICH with 63% sensitivity, 66% specificity, and positive and negative predictive values of 0.91 and 0.25 respectively. Using a 90% probability would increase the model’s specificity to 92%, meaning less risk of placing ICP monitors in patients who do not develop ICH, but would also decrease sensitivity to 34%, meaning that a significant proportion of children who go on to develop ICH may not get identified. Our findings are consistent with those of a study by Forsyth et al. in which a GCS of 8 or less and the presence of a brain CT abnormality separately predicted ICH with sensitivity of 80% and 91%, and specificity of 55% and 38%, respectively [8]. In their model, only the presence of diffuse axonal injury was reliably associated with ICH, and the effect of secondary insults (hypoxia and hypotension) was not tested. It was not clear why female sex was associated with an increased risk of ICH; we did not find any statistical differences between male and female patients with respect to mechanism of injury, ISS, or admission GCS to account for the observed increased risk. A larger prospective study would be needed to corroborate this finding. Finally, in the decision of whether to undergo invasive ICP monitoring, one should consider the low risk of ICP monitor placement in contrast to the potential improvement in outcomes from avoiding ICH and cerebral herniation and maintaining adequate CPP from ICP-guided care [20, 24, 25, 29, 30].
Study limitations
Our data has several limitations. Importantly, this is a retrospective study of local clinical practice of patients who had ICP monitors placed, modifying factors such as brain CT findings, clinical judgment, and nonsurvivable injury, or improving neurological status may alter decisions to place ICP monitors [31]. Over the 10-year time span of the study, medical variations in patient care that resulted in changes in practice also may have occurred. A larger sample size may allow incorporation of more variables, and better determine the relevance of these risk factors, particularly sex. Another limitation is the use of the GOS as an outcome variable which was established for adults. In addition, hospital discharge outcome was used in our analysis but this likely does not reflect a patient’s ultimate outcome as many patients will improve over time after hospital discharge [32]. While both centers are large tertiary academic level 1 trauma centers and practiced according to published guidelines for sTBI in children, we could not account for individual neurosurgical and critical care decisions that may have introduced variation in which patients were selected for ICP monitoring. Finally, we only examined the initial head CT, prior to ICP monitor placement; therefore, we could not exclude the possibility that small new hemorrhages were associated with ICP monitoring device placement.
Conclusions
In this retrospective study, we found a high rate of ICH and low rates of device-related morbidity in patients undergoing invasive ICP monitoring with a GCS of 8 or less and brain CT abnormality. We found that female patients, those with a higher injury severity score and patients who sustained hypoxia prior to PCIU admission, were at greater risk for developing ICH. Our study only highlights the high incidence of elevated ICP requiring intervention in monitored children after sTBI and was not designed to assess any outcome benefit to treatment of ICH episodes directed by the use of the ICP monitor.
References
Bennett TD, Dixon RR, Kartchner C, DeWitt PE, Sierra Y, Ladell D, Kempe A, Runyan DK, Dean JM, Keenan HT (2016) Functional status scale in children with traumatic brain injury: a prospective cohort study. Pediatr Crit Care Med 17:1147–1156
Murphy S, Thomas NJ, Gertz SJ, Beca J, Luther JF, Bell MJ, Wisniewski SR, Hartman AL, Tasker RC (2017) Tripartite stratification of the Glasgow Coma Scale in children with severe traumatic brain injury and mortality: an analysis from a multi-center comparative effectiveness study. J Neurotrauma
Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, Warden CR, Wright DW, American Association for Surgery of T, Child Neurology S, International Society for Pediatric N, International Trauma A, Critical Care S, Society of Critical Care M, World Federation of Pediatric I, Critical Care S (2003) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med 4:S1–S75
Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR, American Academy of Pediatrics-Section on Neurological S, American Association of Neurological Surgeons/Congress of Neurological S, Child Neurology S, European Society of P, Neonatal Intensive C, Neurocritical Care S, Pediatric Neurocritical Care Research G, Society of Critical Care M, Paediatric Intensive Care Society UK, Society for Neuroscience in A, Critical C, World Federation of Pediatric I, Critical Care S (2012) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med 13(Suppl 1):S1–S82
Kochanek PM, Tasker RC, Carney N, Totten AM, Adelson PD, Selden NR, Davis-O'Reilly C, Hart EL, Bell MJ, Bratton SL, Grant GA, Kissoon N, Reuter-Rice KE, Vavilala MS, Wainwright MS (2019) Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, third edition: update of the brain Trauma Foundation Guidelines, executive summary. Pediatr Crit Care Med 20:280–289
Bennett TD, DeWitt PE, Greene TH, Srivastava R, Riva-Cambrin J, Nance ML, Bratton SL, Runyan DK, Dean JM, Keenan HT (2017) Functional outcome after intracranial pressure monitoring for children with severe traumatic brain injury. JAMA Pediatr 171:965–971
Alkhoury F, Kyriakides TC (2014) Intracranial pressure monitoring in children with severe traumatic brain injury: National Trauma Data Bank-Based Review of Outcomes. JAMA Surg 149:544–548
Forsyth RJ, Parslow RC, Tasker RC, Hawley CA, Morris KP, Group UKPTBIS, Paediatric Intensive Care Society Study G (2008) Prediction of raised intracranial pressure complicating severe traumatic brain injury in children: implications for trial design. Pediatr Crit Care Med 9:8–14
Bailey BM, Liesemer K, Statler KD, Riva-Cambrin J, Bratton SL (2012) Monitoring and prediction of intracranial hypertension in pediatric traumatic brain injury: clinical factors and initial head computed tomography. J Trauma Acute Care Surg 72:263–270
Figaji AA, Zwane E, Fieggen AG, Peter JC, Leroux PD (2008) Acute clinical grading in pediatric severe traumatic brain injury and its association with subsequent intracranial pressure, cerebral perfusion pressure, and brain oxygenation. Neurosurg Focus 25:E4
Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA, Group UKPTBIS, Paediatric Intensive Care Society Study G (2006) Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive Care Med 32:1606–1612
Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW (2005) Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery 57:1173–1182 discussion 1173-1182
Liesemer K, Riva-Cambrin J, Bennett KS, Bratton SL, Tran H, Metzger RR, Bennett TD (2014) Use of Rotterdam CT scores for mortality risk stratification in children with traumatic brain injury. Pediatr Crit Care Med 15:554–562
Vavilala MS, Kernic MA, Wang J, Kannan N, Mink RB, Wainwright MS, Groner JI, Bell MJ, Giza CC, Zatzick DF, Ellenbogen RG, Boyle LN, Mitchell PH, Rivara FP, Pediatric Guideline A, Outcomes S (2014) Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med 42:2258–2266
Grinnon ST, Miller K, Marler JR, Lu Y, Stout A, Odenkirchen J, Kunitz S (2012) National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods. Clinical trials (London, England) 9:322–329
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Heinze G, Wallisch C, Dunkler D (2018) Variable selection - a review and recommendations for the practicing statistician. Biometrical journal Biometrische Zeitschrift 60:431–449
Sarnaik A, Ferguson NM, O'Meara AMI, Agrawal S, Deep A, Buttram S, Bell MJ, Wisniewski SR, Luther JF, Hartman AL, Vavilala MS (2018) Age and mortality in Pediatric severe traumatic brain injury: results from an international study. Neurocrit Care 28:302–313
Steyerberg EW, Eijkemans MJ, Harrell FE Jr, Habbema JD (2001) Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making : Int J Soc Med Decis Making 21:45–56
Farahvar A, Gerber LM, Chiu YL, Carney N, Hartl R, Ghajar J (2012) Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg 117:729–734
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3:32–35
Alemayehu D, Zou KH (2012) Applications of ROC analysis in medical research: recent developments and future directions. Acad Radiol 19:1457–1464
Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, Manley GT (2008) Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg 109:678–684
Farahvar A, Gerber LM, Chiu YL, Hartl R, Froelich M, Carney N, Ghajar J (2011) Response to intracranial hypertension treatment as a predictor of death in patients with severe traumatic brain injury. J Neurosurg 114:1471–1478
Pineda JA, Leonard JR, Mazotas IG, Noetzel M, Limbrick DD, Keller MS, Gill J, Doctor A (2013) Effect of implementation of a paediatric neurocritical care programme on outcomes after severe traumatic brain injury: a retrospective cohort study. The Lancet Neurology 12:45–52
Guiza F, Depreitere B, Piper I, Citerio G, Chambers I, Jones PA, Lo TY, Enblad P, Nillson P, Feyen B, Jorens P, Maas A, Schuhmann MU, Donald R, Moss L, Van den Berghe G, Meyfroidt G (2015) Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 41:1067–1076
Alali AS, Gomez D, Sathya C, Burd RS, Mainprize TG, Moulton R, Falcone RA Jr, de Mestral C, Nathens A (2015) Intracranial pressure monitoring among children with severe traumatic brain injury. J Neurosurg Pediatr 16:523–532
Bennett TD, Riva-Cambrin J, Keenan HT, Korgenski EK, Bratton SL (2012) Variation in intracranial pressure monitoring and outcomes in pediatric traumatic brain injury. Arch Pediatr Adolesc Med 166:641–647
Tasker RC (2013) Brain vascular and hydrodynamic physiology. Semin Pediatr Surg 22:168–173
Tasker RC (2014) Intracranial pressure and cerebrovascular autoregulation in pediatric critical illness. Semin Pediatr Neurol 21:255–262
Roumeliotis N, Pettersen G, Crevier L, Emeriaud G (2015) ICP monitoring in children: why are we not adhering to guidelines? Childs Nerv Syst 31:2011–2014
Slovis JC, Gupta N, Li NY, Kernie SG, Miles DK (2018) Assessment of recovery following Pediatric traumatic brain injury. Pediatr Crit Care Med 19:353–360
Acknowledgments
Cynthia Greenwell at Children’s Medical Center Dallas and Tina Day and Lori Barganier at Washington University contributed to data collection.
Funding
This study was supported by the St. Louis Children’s Hospital Foundation, the Sean Glanvill Foundation, the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program (JAP), and The Perot Brain and Nerve Injury Center (DKM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that no competing financial interests exist.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miles, D.K., Ponisio, M.R., Colvin, R. et al. Predictors of intracranial hypertension in children undergoing ICP monitoring after severe traumatic brain injury. Childs Nerv Syst 36, 1453–1460 (2020). https://doi.org/10.1007/s00381-020-04516-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-020-04516-7