Abstract
Introduction
Shunting for hydrocephalus can lead to improvement in the quality of life although the latter has been subdued by complications like shunt infection. Established protocols have contributed to the reduction of ventriculoperitoneal shunt (VPS) infections. Previously, we retrospectively demonstrated a low infection rate despite some of the protocol recommendations not being implemented. The aim of this study was to prospectively establish the incidence of shunt infection in the early post-shunt period following our protocol and elucidate on associated risk factors.
Patients and methods
A multicenter prospective descriptive cohort study of consecutive 209 under-5 children requiring VPS for hydrocephalus was conducted between January 2013 and November 2018. An innovative protocol insisting on intermittent application of povidone-iodine on the skin during the operation was implemented. The patients were followed-up for 3 months post-surgery.
Results
Included were 211 VPS procedures performed on 209 children. The median age was 9 months and 84 were males. Hydrocephalus was non-communicative in 72.0% and aqueductal stenosis was its most frequent cause (84.9%). Most surgeries were performed in the morning (90.5%), electively (95.3%), and for the first time (91%). The median duration of surgery was 65 min. Shunt infection rate was 1.9% (n = 4) (95% CI 0.7 to 5.0%) per procedure.
Conclusion
The observed infection rate was low. This suggests that the protocol followed captured the most critical components necessary to ensure low infection rates and that simple measures implemented in economically challenged environments may achieve internationally acceptable infection rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is the most frequent neurosurgical problem encountered in children in our setting [10] and worldwide [17]. Ventriculoperitoneal shunt (VPS) is a common neurosurgical procedure in the therapeutic armamentarium for hydrocephalus [15]. Although the resurgence of endoscopic third ventriculostomy (ETV) with its advantages is an appealing alternative [3], conventional CSF shunting is still a treatment option with excellent prognosis in many hydrocephalic patients [17]. However, the gains in the quality of life after shunting are often subdued by significant failure rates from complications like malfunction and shunt infection [29] and dissatisfaction from their long-term morbidity. Mortality rates are still elevated ranging from 10 to 15% [18] to as high as 30–40% [8, 19]. The risk of subsequent morbidity leads to increase in resources spent to treat a potentially preventable illness against a background of limited resources in Africa.
Several established protocols have repeatedly contributed to reduction of VPS infections [2, 12, 13, 20, 25,25,27] yet “zero infection” is still a far cry. With reported risk of early infection after shunt surgery ranging from 3 to 20% [2, 7, 14, 23, 33], current acceptable infection rates are less than 5–7% [18]. Fifty percent of shunt infections occur within 2 weeks post-shunt [18], 70% [2] to 90% [9] within 30 days, and more than 90% within 6 months [1, 2, 21] post-surgery; most being staphylococcal infections from the skin [8, 18]. Therefore, shunt infection in most cases is a complication of shunt surgery [2] and could/should be mitigated and averted.
Previously, we [22] reported low infection rates of 3.5% per procedure by rigidly adhering to some of the recommendations of infection minimizing protocols [2, 26]. Because not the entirety of the recommendations was implemented, our results suggested that some of the measures in the protocols have minimal or no effect on infection reduction hence the need for adapted protocols tailored to our setting to avoid future irrational use of scarce resources. The fact that the exact causes or the relative contribution of identified risk factors remains unascertained necessitates the identification of factors associated with shunt infection locally.
The purpose of this study was thus to prospectively establish the incidence of shunt infection in the early post-shunt period at three separate countries where our protocol was implemented, and elucidate on associated risk factors.
Patients and methods
Study design, setting, and period
This was a non-concurrent, multicenter, prospective descriptive cohort study [6] of consecutive 209 under-5 children requiring VPS for hydrocephalus. The children were recruited from three countries (Zimbabwe, Namibia, and Democratic Republic of Congo) and prospectively observed and followed-up from admission for surgery to 3 months post-surgery during the period of January 1, 2013, to November 30, 2018. The initial cohort study was conducted at two university teaching hospitals in Harare (the only institutions with pediatric neurosurgery service in Zimbabwe) and the protocol replicated in Oshakati (Namibia) and Lubumbashi (DRC) hospitals.
Study population
Sampling and recruitment
Consecutive patients were entered into the study on the day of shunt insertion. Informed consent forms were signed by the consenting parent or guardian of study subjects.
Inclusion criteria:
Patients with hydrocephalus requiring VP shunt surgery
Patients aged 5 years or less on the day of shunting
Exclusion criteria:
Patients with hydrocephalus treated by alternative diversion instead of VPS
Patients with pre-existing CSF infection on culture of intra-operatively collected CSF sample
Sample size justification
We planned a prospective cohort study on children with hydrocephalus for whom VPS was done with “no control group.” Prior data indicated infection rate after VPS surgery of 4% [2]. Applying Dobson’s formula for sample size calculation [4] and adjusting for a 5% contingency, the minimum required sample for the study was 62 subjects. A total of 68 children were included in the pilot cohort study (Harare), 94 cases in Oshakati, and 47 children in Lubumbashi hospitals.
Procedure (patient management)
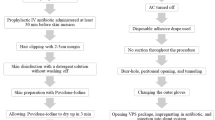
Patients with hydrocephalus indicated for shunting were booked for surgery after anesthetic clearance. They were operated through the classic VP Shunt insertion technique and the protocol adopted for shunt implantation is as in Table 1.
Measurement of variables
Prematurity was defined as a gestational age of less than 36 weeks from last normal menstrual period or ultrasound determined estimated gestational age less than 36 weeks at delivery. The nutritional status of the children was assessed by examination of their “Road to Health Card (RTHC)” [31].
Outcome measure
Primary outcome was shunt infection at 3 months defined in accordance with the consensus recommendations of the Canadian Pediatric Neurosurgery Study Group [5]. The criteria to diagnose surgical site infection followed the 1999 Centre for Disease Control guidelines [16].
Operating time was calculated from the first skin incision to completion of wound dressing.
Follow-up
Follow-up was from the time of admission for shunt insertion to 3 months after the shunting operation. The first review was at day 10, then at 6 weeks, and finally at 3 months post-operation during which they were checked for shunt infection in addition to classic clinical routines. When necessary, telephone calls were made to remind parents of pending outpatient review dates.
Data collection
Data was collected by the respective country investigators using a questionnaire designed and adapted for the study.
Statistical analysis
Analysis was done using STATA/MP version 13.0.
Ethical clearance
Authority to perform the study was obtained from the Joint Research and Ethics Committees of the hospitals.
Results
A total of 211 shunt insertion procedures performed on 209 children were analyzed. One male and female had 2 separate shunt insertion procedures during the study period. There was one loss to follow-up (Fig. 1).
The median age of the participants was 9 months (p25 = 6 months p75 = 14 months) (min. 2 months and max. 59 months).
There were 84 males and 125 females and were all Africans. Two patients (2.56%) were delivered prematurely and had no antecedent history of surgery to treat hydrocephalus secondary to intraventricular hemorrhage of prematurity.
The mean weight ± standard deviation (SD) was 8.76 ± 2.58 kg. The majority (n = 180 of 209) of children had a normal growth trajectory (between ± 2 SD lines). Twenty-seven (21.0%) had a weight-for-age below 2 SD. Breast-feeding was documented in 109 (95.2%) children (Table 2).
Type and etiology of hydrocephalus
Hydrocephalus was non-communicating in 72.0% (n = 152). Aqueductal stenosis was the most frequent cause for non-communicating hydrocephalus in 84.9% (n = 129). Post-infectious HCP accounted for 44.1% (n = 26) of communicating HCP cases (Table 2).
Operative factors
The median pre-operative hospital stay was 1 day (IQR 1–2 days). All the children had Chhabra shunts (Surgiwear Ltd., India) implanted using the protocol.
In 90.5% children (n = 191), the surgeries were performed in the morning. In 4.7% of the shunts, the surgery was done emergently. Most children (91%) were having their first shunt implanted.
The median duration of surgery was 65 min (IQR from 60 to 85 min). The mean number of persons in the theater during the operations was 8.22 (SD 2.86) (for Harare) (Fig. 2).
For Harare, 57 (71.3%) of the shunts were done by surgeons in their 3rd year of surgical training, and 7 (8.8%) by a consultant neurosurgeon. For the other two centers, a consultant neurosurgeon did all the surgeries (Table 3).
Outcome
Shunt infection was diagnosed in four children giving an infection rate of 1.9% (n = 4) (95% CI 0.7 to 5.0%) per procedure. The infection rate was 2.94% in Harare, 2.13% in Oshakati, and 0% in Lubumbashi hospital. The median time to develop infection was 8.5 days (p25 = 5 and p75 = 12) (Fig. 3).
Case no. 1: A 2-month old with post-infectious communicating hydrocephalus. The infecting organism isolated from both peri-shunt tissues and CSF from a shunt tap was Staphylococcus aureus. The clinical findings were a peri-subcutaneous shunt path abscess which complicated into meningitis and ventriculitis. This infection developed 10 days after shunt implantation.
Case no. 2: A 7-month old, with congenital communicating hydrocephalus. A mixed infection consisting of gram-negative rods of both lactose fermenting and non-lactose fermenting coliforms was causal. Presentation of this infection was 2 weeks from shunt implantation.
Case no. 3 was cases of post-infectious hydrocephalus and case 4 Chiari type 2.
They were respectively 3 and 4 months old. Staphyloccocus aureus was detected in both cases. The children were treated by removal of the shunt, insertion of external ventricular drainage, and sensitivity-guided antibiotic therapy followed by successful reimplantation of the VPS.
Other post-operative complications
A temporary post-operative CSF leaks developed in 2 children and a stitch abscess in one other child (not the children with shunt infections). CSF leak resolved spontaneously and stitch abscess cleared with stitch removal and application of anti-staphylococcal ointment.
Risk factors for shunt infection
The low number of established infections in this study precluded the statistical subgroup analysis of risk factors to allow identification of local risk factors.
Discussion
Hydrocephalus is a common neurosurgical problem in the pediatric age group [10] [28]. VPS is the mainstay in the treatment of a significant proportion of children with hydrocephalus. Among VPS complications, infection continues to be a serious cause of shunt failure; is the major source of morbi-mortality, an important contributor to the cost of care and a true frustrating problem for clinicians [1].
Several protocols have been developed and implemented over the years to curb shunt infection [2, 12, 13, 20, 22, 26, 27] but only very few centers in the world have achieved infection rate below 1% [28]. These centers employ precautions that are difficult to replicate in most hospitals especially in developing countries and teaching hospitals with trainees [1].
Following the strict adoption and implementation of our aforementioned protocol (Table 1), we observed an infection rate of 1.9% per procedure validating the previous retrospective established rate of 3.5% [22] in the same setting (Table 1). Our findings mirror those of Mottolese et al. (infection rate of 2.8%) [20], albeit higher than those of Choux et al. (0.17%) [2] and Pirotte et al. (< 1%) [26] but favorably comparable with those of Rotim et al. (5.3%) [27], Kestle et al. (3.8%) [12], and 5.7% for hospitals forming the Hydrocephalus Clinical Research Network Quality Improvement Initiative [13]. Sibanda et al. in 1991 had reported a rate of 13.2% in a period where there was no documented protocol [30].
Our observed rate in between the low outliers and the lower figure of world averages challenges the notion that these low rates can only be achieved in well-resourced settings as suggested by their physical location. The common thread running through all these low outliers is the application of institution-specific peri-operative protocols culminating to reduction in shunt infection rates.
The rationale behind these protocols has been the realization that most shunt infections occur within 3 months post-surgery thus are a result of peri-operative factors which could be mitigated by the application of these protocols [2]. Varied as these protocols are, a common theme found among them is the need for limited shunt hardware handling, delayed opening of the shunt prior to implantation, and avoidance of contact of shunt with skin. All other measures instituted vary from protocol to protocol (Table 4).
Some of the measures are difficult to implement in low-income settings as ours, viz limited operating room traffic, skin preparation with chlorhexidine, and choice of surgeon. However, the effectiveness of our protocol suggests that despite our resource restraints, the protocol captured the critical elements necessary to have a significant impact on the infection rate.
The limitation of persons in theater and restriction of traffic into and out of theater is difficult to implement because our institution is a university training center (for Harare). In the absence of intraoperative audiovisual aids to demonstrate procedures, it is customary to have numerous students in theater taking turns to observe proceedings during shunt implantation (Fig. 3); a scenario contrary to the recommendations of other protocols [2, 12, 13, 20, 26, 27].
Another notable deviation from international norms is the continued use of 10% povidone-iodine solution to prepare the patient’s skin prior to draping and its intermittent reapplication intraoperatively instead of chlorhexidine solutions. It has been well established that bacterial skin recolonization from the sweating gland occurs after about 30 to 45 min. Chlorhexidine has been proven to be a better skin antiseptic with a more durable action [28]. Our protocol required that 10% povidone-iodine solution be intermittently applied to keep the peri-incisional skin of the operative sites moist to ensure continued suppression of skin microbial contaminants. Thus, chlorhexidine’s superiority over 10% povidone-iodine solution’s clinical relevance is brought into question as a low infection rate using the less than ideal solution was still achieved. The inference to be made is that the purported inferior action of 10% povidone-iodine solution is still effective enough to ensure low infection rates when combined with meticulous surgical technique. Povidone-iodine (10%) solution therefore seems to be a reasonable alternative when chlorhexidine is either not readily available or unaffordable.
Our average duration of surgery was longer than the recommended time of surgery for protocols in institutions having an infection rate of less than 1% and all but 7 of the procedures were performed by consultants (for Harare). This is in stark contrast with the Choux and the Pirotte protocols where the procedures were done by a senior neurosurgeon and a single senior neurosurgeon, respectively [2, 26]. Despite our observed rate not as low as that reported by them, the pivotal role played by the observance of surgical technique is emphasized in reducing occurrence of infections [2, 26]. With the foregoing considered the corresponding influence of seniority on the development of shunt infection is subsequently subdued.
It was noted that 95.2% of the children were breast-feeding, a factor that may have positively influenced the results obtained in accord with the protective effect of breast-feeding for children under the age of 6 months [24]. However, our study population was generally older (p50 = 9 months).
Another peculiarity of this study was the positive effect on shunt infection by the short pre-surgery and post-surgery hospital stays. A more aggressive approach of discharging patients between day 2 and 4 post-operatively was adopted in our protocol that may have contributed to our lower infection rates.
An expected preponderance of congenital and post-infectious hydrocephalus in a developing setting formed the study population. There were 17 children in our study with myelomeningocele, an unexpected low number compared with a previous study in the same setting [30]. Given the establishment of myelomeningocele as a risk factor for shunt infection, the inclusion of a higher proportion of subjects with this risk factor inter alia the absence of a set protocol possibly explained the higher infection rates of 13.2%; they [30] observed and a lower infection rate (1.9%) in this study.
Non-measured factors worth mentioning as they may have positively influenced the results obtained are the education about shunt infection that was routinely given to caregivers during informed consent. Additionally, reciprocal phone calls between the principal investigator and parents, the closer follow-up, may have led to more vigilance and prevention of infection beyond the immediate peri-operative period. This period was noted to be a time when the patients would still be vulnerable to contamination leading to shunt infection [32].
A closer look at the 4 children with shunt infection shows that three were undernourished, 2 had post-infectious hydrocephalus and another Chiari type 2. The aforementioned considered together put these children at an inherently increased risk of shunt infection. Furthermore, the development of the infections within a month of surgery makes them direct complications of surgery. The cases of infection were potentially avoidable if surgery had been delayed to optimize the patients.
The strength of our study was in its design: Our cohort study just like that of Pirotte et al. [26] and Kestle et al. [13] was prospective providing level 3 evidence. Previous protocols were of a lower level of evidence (level 4) because they were retrospective and used historical controls [2, 12, 20, 26, 27]. Selected comprehensive protocols for shunt infection control in children [2, 12, 13, 20, 26, 27] are summarized in Table 4.
Study limitations: The main limiting factor of the study was the sample size given the low infection rate obtained that did not allow for statistical analysis and verification of risk factors. As a result, no justifiable modification of the protocol can be made based on the findings of this study. Adjunctive anthropometric measurements of the nutritional state of these hydrocephalic children such as the mid-arm circumference and skinfold thickness combined with clinical assessments of the skin, hair, and muscle bulk would have been more appropriate. These adjuncts would expose falsely normal or accelerated growth curves due to pathologically increasing CSF as opposed to general body weight gain. This effect was not catered for in the study.
The durability of the results after the 3-month follow-up compared with the lengthier follow-ups in other series may be questioned. However, the findings that 90% of VPS infections occur within 30 days [9] and that a high-risk pediatric population was singled out for analysis mitigate the shortcoming of the short follow-up.
Conclusion
The institution of a protocol that emphasizes meticulous surgical technique and adherence to antisepsis reduces infection rate comparable with those from well-resourced settings. The main paradigm shift is the acceptance to work in a wet environment of povidone-iodine which eliminates bacterial recolonization of the skin. Continued data collection is recommended as it will allow identification of locally significant risk factors. This information will facilitate the modification of the protocol to control for the factors and assist in further reduction of shunt infections and their sequelae.
Abbreviations
- CSF:
-
Cerebrospinal fluid
- VPS:
-
Ventriculoperitoneal shunt
- ETV:
-
Endoscopic third ventriculostomy
- EVD:
-
External ventricular drainage
- HCP:
-
Hydrocephalus
References
Akhaddar A (2015) Complications of extrathecal CSF shunts:infective complications. In: Di Rocco C, Turgut M, Jallo G, Martínez-Lage JF (eds) Complications of CSF shunting in hydrocephalus: prevention, identification, and management. Springer International Publishing, Cham, pp 141–149
Choux M, Genitori L, Lang D, Lena G (1992) Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 77:875–880
Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs endoscopic third ventriculostomy in infants: are there different types and/or rates of complications? A review. Childs Nerv Syst 22:1573–1589
Dobson AJ (1984) Calculating sample size. Trans Menzies Foundation 7:75–79
Drake JM, Singhal A, Kulkarni AV, DeVeber G, Cochrane DD (2012) Consensus definitions of complications for accurate recording and comparisons of surgical outcomes in pediatric neurosurgery. J Neurosurg Pediatr 10:89–95
Esene IN, Ngu J, Elzoghby M, Solaroglu I, Sikod AM, Kotb A, Dechambenoit G, ElHusseiny H (2014) Case series and descriptive cohort studies in neurosurgery: the confusion and solution. Childs Nerv Syst 30:1321–1332
Gathura E, Poenaru D, Bransford R, Albright AL (2010) Outcomes of ventriculoperitoneal shunt insertion in Sub-Saharan Africa. J Neurosurg Pediatr 6:329–335
George R, Leibrock L, Epstein M (1979) Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg 51:804–811
Gutierrez-Murgas Y, Snowden JN (2014) Ventricular shunt infections: immunopathogenesis and clinical management. J Neuroimmunol 276:1–8
Kalangu KK (2000) Pediatric neurosurgery in Africa--present and future. Childs Nerv Syst 16:770–775
Kalangu KKN, Esene IN, Dzowa M, Musara A, Ntalaja J, Badra AK (2019) Towards zero infection for ventriculoperitoneal shunt insertion in resource-limited settings: a multicenter prospective cohort study. Childs Nerv Syst. https://doi.org/10.1007/s00381-019-04357-z
Kestle JR, Hoffman HJ, Soloniuk D, Humphreys RP, Drake JM, Hendrick EB (1993) A concerted effort to prevent shunt infection. Childs Nerv Syst 9:163–165
Kestle JR, Riva-Cambrin J, Wellons JC III, Kulkarni AV, Whitehead WE, Walker ML, Oakes WJ, Drake JM, Luerssen TG, Simon TD, Holubkov R (2011) A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr 8:22–29
Komolafe EO, Adeolu AA, Komolafe MA (2008) Treatment of cerebrospinal fluid shunting complications in a Nigerian neurosurgery programme. Case illustrations and review. Pediatr Neurosurg 44:36–42
Limbrick DD Jr, Baird LC, Klimo P Jr, Riva-Cambrin J, Flannery AM (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J Neurosurg Pediatr 14(Suppl 1):30–34
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–278
Mark R.Lantosca , James M. Drak: Cerebrospinal fluid shunts, in A. Leland Albright, Ian F. Pollack (eds): Operative techniques in pediatric neurosurgery. New York, Thieme, 2001, pp 3–14
Greenberg MS (2016) Ch. 21:Skull, spine, and post-surgical infections: 21.1 shunt infection. In: Greenberg MS (ed) Handbook of neurosurgery. Thieme, New York, pp 339–342
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 36:858–862
Mottolese C, Grando J, Convert J, Abdoulrahman M, Lelievre H, Vandenesch F, Bret P, Lapras C (2000) Zero rate of shunt infection in the first postoperative year in children--dream or reality? Childs Nerv Syst 16:210–212
Moussa WM, Mohamed MA (2016) Efficacy of postoperative antibiotic injection in and around ventriculoperitoneal shunt in reduction of shunt infection: a randomized controlled trial. Clin Neurol Neurosurg 143:144–149
Musara A, Kalangu K (2008) Ventriculperitoneal shunt infection in pediatric age group in Harare, Zimbabwe. Childs Nerv Syst 24:1280
Mwachaka PM, Obonyo NG, Mutiso BK, Ranketi S, Mwang’ombe N (2010) Ventriculoperitoneal shunt complications: a three-year retrospective study in a Kenyan national teaching and referral hospital. Pediatr Neurosurg 46:1–5
Nejat F, Tajik P, Ghodsi SM, Golestan B, Majdzadeh R, Yazdani S, Ansari S, Dadmehr M, Ganji S, Najafi M, Farahmand F, Moatamed F (2008) Breastfeeding: a potential protective factor against ventriculoperitoneal shunt infection in young infants. J Neurosurg Pediatr 1:138–141
Parker SL, McGirt MJ, Murphy JA, Megerian JT, Stout M, Engelhart L (2015) Comparative effectiveness of antibiotic-impregnated shunt catheters in the treatment of adult and pediatric hydrocephalus: analysis of 12,589 consecutive cases from 287 US hospital systems. J Neurosurg 122:443–448
Pirotte BJ, Lubansu A, Bruneau M, Loqa C, Van CN, Brotchi J (2007) Sterile surgical technique for shunt placement reduces the shunt infection rate in children: preliminary analysis of a prospective protocol in 115 consecutive procedures. Childs Nerv Syst 23:1251–1261
Rotim K, Miklic P, Paladino J, Melada A, Marcikic M, Scap M (1997) Reducing the incidence of infection in pediatric cerebrospinal fluid shunt operations. Childs Nerv Syst 13:584–587
Sarmey N, Kshettry VR, Shriver MF, Habboub G, Machado AG, Weil RJ (2015) Evidence-based interventions to reduce shunt infections: a systematic review. Childs Nerv Syst 31:541–549
Sciubba DM, Noggle JC, Carson BS, Jallo GI (2008) Antibiotic-impregnated shunt catheters for the treatment of infantile hydrocephalus. Pediatr Neurosurg 44:91–96
Sibanda EN, Levy LF, Makarawo S (1991) Infection after Harare valve V-P shunt operations: a review of 92 cases. Cent Afr J Med 37:397–403
Tarwa C, De Villiers FP (2007) The use of the road to health card in monitoring child health. S Afr Fam Pract 49:15–15d
Thompson DN, Hartley JC, Hayward RD (2007) Shunt infection: is there a near-miss scenario? J Neurosurg 106:15–19
Warf BC (2005) Comparison of 1-year outcomes for the Chhabra and Codman-Hakim Micro Precision shunt systems in Uganda: a prospective study in 195 children. J Neurosurg 102:358–362
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Patient consent
The patient or guardian has consented to this study by signing the consent form.
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalangu, K.K.N., Esene, I.N., Dzowa, M. et al. Towards zero infection for ventriculoperitoneal shunt insertion in resource-limited settings: a multicenter prospective cohort study. Childs Nerv Syst 36, 401–409 (2020). https://doi.org/10.1007/s00381-019-04357-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04357-z