Abstract
Purpose
Pediatric glioblastoma (pGBM) tumors have been identified as an entity distinct and different from the adult variety of GBM not only with respect to pathogenesis, genetics, and molecular alterations but also in clinical outcomes and overall survival. This study aims to evaluate the immunohistochemical profile of molecular markers in pediatric GBM and correlate them with clinical features and prognosis.
Materials and methods
We retrospectively analyzed 29 pGBMs (age range 3 to 18 years), operated at our institute between 2009 and 2014, and evaluated their clinical and histopathological features along with the immunohistochemical expression of clinically relevant molecular markers: H3K27M, p53, ATRX, and IDH1 (R132H), and correlated their expression with clinical features. We further assessed the prognostic value of these markers in our cohort of patients.
Results
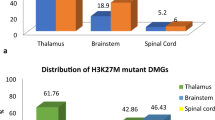
The median overall survival (OS) of the cohort was 6.00 ± 0.882 months. The mean overall survival was 7.571 ± 1.118 months which was lower than in most studies. Preoperative Karnofsky Performance Score (KPS), extent of surgical resection, and adjuvant radiotherapy were found to be the clinical factors strongly influencing median survival (p < 0.05). Loss of ATRX expression was predominantly noted in hemispheric tumors (84%), while p53 staining was maximum in thalamic tumors (8 out of 9 cases). H3K27M mutant protein expression was noted in 8/9 thalamic tumors and 5/7 tumors in the brain stem-cerebellar-peduncular region. Patients with tumors showing H3K27M immunopositivity had the worst prognosis with a mean OS of 5 months ± 0.832 months, as against patients with H3K27M-immunonegative tumors, which was 10.143 ± 1.866 months(p = 0.006). Other markers like p53, ATRX, and IDH1 did not influence the prognosis in this patient cohort. ATRX loss of expression was associated with a better OS, with a trend to significance, and such an association has not been reported earlier.
Conclusions
Ours is one among the few studies from India describing the clinical parameters and evaluating the key immunohistochemical markers in pGBM and deriving their prognostic significance. The study reiterates the poor prognostic significance of H3K27M immunopositivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a paucity of literature with respect to pediatric glioblastoma (pGBM) when compared with the abundance of information available describing the adult counterpart. Studies on pGBM have reported the incidence to be around 3% of all pediatric central nervous system (CNS) tumors [1, 2]. The Central Brain Tumor Registry (CBTRUS) data in 2013 showed that among children and adolescents aged 0–19, malignant glioma accounted for 11.5% of all tumors and pGBM constituted 2.9% of these tumors. The incidence of pediatric GBM in India can be understood from the various hospital-based published studies. Das et al. noted that pediatric GBM constituted nearly 5.5% of all GBMs in their series [3]. Another Indian series reported the percentage of pediatric cases among all GBMs to be around 8.5% [4]. A previous study from our institute had shown that out of a total of 694 pediatric intracranial tumors diagnosed over a period of 5 years, pGBM accounted for 5.8% of cases [5].
In the last few years, studies on the molecular signatures specific to pGBM have revealed that these tumors are different from their adult counterparts in terms of their genomic landscape. The histone H3 mutations have been recently identified to play a significant role in pGBM pathogenesis [6]. However, clinical and histomolecular studies that delineate their pathobiology are few in number.
With this background, we evaluated the clinical and histopathological features of pGBM along with the immunohistochemical expression of clinically relevant molecular markers: H3K27M, p53, ATRX, and IDH1 (R132H), and correlated their expression with clinical features. We further assessed the prognostic value of these markers in our cohort of patients.
Materials and methods
This retrospective study was carried out on pGBM cases (aged 18 years or less) managed at our institute during the period of 5 years (2009–2014). The relevant clinical data were obtained from the case records. Patients were followed up as part of the standard treatment and the status of the patient at last follow-up was documented. The study has been approved by our “Institute Ethics committee.”
Pathology review
Hematoxylin and eosin (H&E)–stained slides and formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved from the Department of Neuropathology. The H&E-stained slides were reviewed by the neuropathologist (VS) for confirmation of diagnosis. Tissue microarrays (TMAs) were constructed for immunohistochemistry.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on 4-μm TMA sections using antibody against the following: H3K27M [1:300, Millipore, USA], H3K27me3 [1:100, Millipore, USA], IDH1-R132H [1:50, Dianova, Germany], ATRX [1:50, Sigma-Aldrich, USA], and p53 [1:100, Dako]. Briefly, following the initial processing steps, the slides were incubated overnight with the primary antibody. This was followed by incubation with a secondary antibody (MACH1, Biocare Medical), and 3,3′-diaminobenzidine (Sigma-Aldrich, USA) was used as chromogenic substrate.. A positive control slide was incorporated with each batch of staining. Cytoplasmic staining of IDH1 (R132H) and nuclear staining of ATRX, p53, and H3K27M were assessed in the tumor cells. Loss of ATRX nuclear immunopositivity was considered surrogate for ATRX mutant status. Strong P53 and H3K27M nuclear staining was considered positive.
Statistical analysis
Statistical analysis was carried out using SPSS (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY; IBM Corp). The data was analyzed for distribution pattern and our data followed a non-parametric distribution. Descriptive analysis was done for all the parameters. Chi-square test was performed for the categorical values of the biomarkers. One-way ANOVA was done to compare continuous variables versus categorical variables. Overall survival with respect to the various variables was assessed using log-rank tests for pooled data, and the Kaplan-Meier curves were generated.
Results
The study included 29 children with 13 boys and 16 girls. The median age of the cohort was 10.00 ± 3.91 years (range 3–18 years). The median duration of symptoms was 1 ± 1.76 months (range 1–6 months). The clinical and radiological features are summarized in Table 1.
T1-weighted post-contrast MRI axial image (a) in a 12-year-old child presenting with raised ICP headache and vomiting, showing a right frontal GBM with peripheral enhancement. Post-op CT head (b) showing a gross total resection. Axial T1-weighted post-contrast MRI image (c) in a 10-year-old child who presented with right upper and lower limb weakness showing a peripherally enhancing lesion in the left thalamus with perilesional edema. Post-op CT scan (d) showing a subtotal resection. Sagittal (e) and axial (f) post-contrast MRI images in a 13-year-old child who presented with lower cranial nerve palsy, showing a non-enhancing dorsal exophytic lesion in the medulla and right cerebellar peduncle
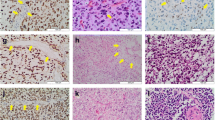
Histology and immunohistochemical profile from a case of pediatric glioblastoma in an 18-year-old patient, located in the left frontal region of the brain. The tumor is composed of undifferentiated and multinucleated astrocytic cells and shows a large area of necrosis (a); tumor cells are strongly GFAP positive (b), show IDH1 (R132H) positivity (c), ATRX loss of expression (d), and P53 positivity (e). The tumor is negative for H3K27M (f) and shows a retained expression of H3Me3 (g). All microphotographs are magnified with × 180
Histology and immunohistochemical profile from a 15-year-old pediatric glioblastoma patient with tumor in the left thalamus (midline) of the brain. The tumor is cellular with an area of necrosis (a) and composed of pleomorphic tumor cells (b). The tumor cells show a diffuse GFAP positivity (c), are negative for IDH1 (R132H) (d), show an ATRX loss of expression (e), and are positive for P53 (f). The tumor cells are positive for H3K27M (g) and show a concomitant reduced H3Me3 expression (h). All microphotographs are magnified with × 180
Surgical management
Out of the 29 tumors, 13 were hemispheric, 9 (31%) were noted in the thalamic region, 5 (12%) in the brain stem, and two tumors were noted in the cerebellar peduncles. Among the hemispheric tumors, 9 (31%) were located in the frontal and 4 (13.8%) in the temporal region. Surgical decompression was performed for tumors, which were accessible. For deep-seated lesions, histological diagnosis was obtained on a stereotactic biopsy. Total or near-total resection could be achieved in 14 patients (48.3%). Twelve patients (41.3%) underwent subtotal resection and three (10.3%) underwent stereotactic biopsy (Fig. 1). Of the thalamic tumors, three underwent near-total resection, five underwent subtotal resection, and one case was biopsied. Most of the thalamic tumors presented with large lesions, with raised intracranial pressure, and therefore, patients underwent craniotomy and decompression of the tumor.
Most of the patients with posterior fossa tumors involved the brain stem and had an exophytic component which was amenable for resection. These patients underwent midline suboccipital craniectomy. Near-total excision was possible in one case while five cases underwent subtotal decompression and one underwent stereotactic biopsy (STB). It was noted that total or near-total resections were more feasible among hemispheric lesions and the same was difficult to achieve with lesions in the thalamus or brain stem. The extent of resection with respect to location of the tumor is depicted in Table 2.
All the patients were followed up as part of the standard care, and their latest status and time of expiry were documented. One patient was lost to follow-up. The remaining patients (n = 28) in the cohort had expired at the time of the last follow-up, out of which two patients expired in the hospital after prolonged stay. All patients were advised of adjuvant therapy. However, only 16 (57.1%) had taken adjuvant radiotherapy (RT). The patients underwent the prescribed adjuvant therapy from different oncology centers close to their residential locations. Out of 16 patients who completed RT, only 13 (44.8%) patients took concomitant temozolomide-based chemotherapy. Unfortunately, 10 (35.7%) patients did not receive any adjuvant therapy in our cohort. Fifteen (53.6%) patients had documented recurrence and all the recurrences occurred at the primary site itself. Only one patient underwent re-exploration and subtotal decompression of recurrent tumor. Others did not undergo re-surgery because of either poor Karnofsky Performance Score (KPS) or parents not willing for any further surgical intervention.
IHC features in pGBM
Out of the 29 cases in our cohort, p53 immunopositivity was noted in 20 (69%) cases. ATRX loss of expression was noted in 9 (31%) cases. IDH1 (R132H) immunopositivity was seen in two (6.9%) cases only. H3K27M nuclear staining was evident in 14 (48.3%) cases. We noted that H3K27M immunopositivity was predominantly found in the tumors in the midline region (13/15; 86.6%), namely the thalamic and brain stem location. Interestingly, one hemispheric tumor was positive for H3K27M. Other markers such as P53, ATRX, and IDH1 (R132H) did not correlate with the location of tumors (Fig. 2, and 3). The location-wise distribution of IHC markers is shown in Table 3.
Summary of survival analysis
Survival analysis was performed using log-rank tests for pooled data and the Kaplan-Meier curves were generated. When median survival was not reached, mean survival value was mentioned. The mean overall survival (OS) for the whole cohort was 7.57 ± 1.12 months. The median OS was 6.00 ± 0.88 months. Various clinical factors like age group, tumor location, extent of resection, adjuvant therapy, and preoperative KPS were analyzed with survival (Table 4). We noted that hemispheric location, total/near-total resection, KPS of 70 and above, and adjuvant therapy were favorable prognostic factors in univariate analysis.
Among the IHC markers, we noted that H3K27M immunopositivity was associated with a worse prognosis (mean OS 5 ± 0.83 months), as against H3K27M-immunonegative tumors (mean OS 10.14 ± 1.87 months) (p = 0.006). Other markers like P53, ATRX, and IDH1 (R132H) did not influence the prognosis in this patient cohort.
Discussion
Glioblastoma in the pediatric age group is known as a cause of significant morbidity and mortality with poor survival rates in general. The present study included 29 pGBM cases managed surgically over a period of 5 years at our institute. Most studies report a definite male preponderance [1, 3, 4] while some showed a female preponderance like ours [2]. The median duration of symptoms of our cohort was 1 ± 1.76 months (range 1–6 months). Other studies have shown a range varying from 1 day to 12 months with a median duration of 2.5 months [7]. In general, pGBM seems to be a rapidly progressive disease with a short duration of symptoms.
In our study, the majority of tumors were located in the cerebral hemispheric region, followed by the thalamus and brain stem. This is in line with previous reported studies that have shown similar localization, with a predominance of hemispheric lesions, frontal being more common than other lobes, along with a lower incidence of tumors in the posterior fossa [3, 7]. Thalamic lesions comprised 30% of the cohort in our study, which is slightly more than the other reported studies.
Our management included surgical decompression for large and accessible tumors, while STB was preferred in deep-seated small lesions. STB would be the preferred procedure in thalamic lesions; however, if the lesions are large, causing midline shift, they can be decompressed through various transventricular approaches, as noted in some other studies [1, 8]. Similarly, for brain stem lesions with exophytic component, surgical decompression was preferred. However, in intrinsic brain stem gliomas, STB for diagnosis or direct radiotherapy would be the option of management. A previous study of STB of brain stem lesions from our institute has shown that GBM/high-grade glioma constituted 29.3% of all the brain stem lesions in children [9].
CCG-943, a randomized study in children, has clearly demonstrated a definite survival advantage associated with adjuvant chemotherapy in GBM. The 5-year event-free survival was 36–56% for RT and chemotherapy compared with 11–25% for RT alone (p < 0.026). Also, children with GBM treated with combination therapy had significantly better outcomes than those treated with RT alone [10].
As mentioned earlier, the number of patients who took RT in our series was only nearly half of the total (16/29). Ten patients out of 29 did not undergo any form of adjuvant therapy despite advising to do so. The lack of adjuvant therapy in a significant part of the cohort will have implications in terms of prognosis and overall survival. Therefore, the survival data from the present study may not represent the optimal/best outcomes that can be achieved in this disease. We do not have information from other series regarding the dropout numbers for adjuvant therapy. Though the present study did not specifically address the various reasons for not undergoing adjuvant therapy, diverse factors could have probably influenced the decision.
The various factors that could influence the default in treatment could be poor educational and socioeconomic backgrounds, prevalent stigma against the ill-effects of radiation therapy, and the exceedingly long waiting periods at centers for adjuvant therapy. Patients who receive delayed appointments for adjuvant treatment may not come back for the same at all, owing to a multitude of problems like financial concerns, lack of understanding of the disease itself, and loss of motivation to continue treatment once the diagnosis of cancer with a poor prognosis has been communicated. The need of the hour in a growing economy like ours is to cater these issues which include improving facilities at centers for adjuvant therapy and decreasing the waiting periods. Education of the parents and care givers and counseling them thoroughly regarding the disease process and the emphasis on the need for complete therapy would help in eradicating the stigma surrounding adjuvant therapy which in turn may decrease dropout rates. The present study highlights this important area where more work is required for optimal management of brain tumors.
Most studies have reported a median OS ranging between 12 and 20 months [3, 8, 11]. Various factors namely good preoperative performance status, complete surgical excision, adjuvant chemotherapy, female sex, location of tumors, and longer duration of symptoms are all known to influence the factors and offer favorable prognosis [1, 2, 12, 13]. The median OS reported in our study is less compared with that in other series, which can be due to various factors like lack of adjuvant therapy in a significant proportion and more incidence of deep-seated lesions, which precludes the extent of resection and a higher proportion of patients with poor preop KPS.
In literature, TP53 mutations have ranged from 25 to 36% in pGBM [14,15,16]. In our study, we performed IHC as surrogate for molecular markers. We noted p53 immunopositivity in 69% of all cases. In a previous report from our group on pediatric GBM, P53 was found to be immunopositive in 53.7% of the tumors, which was predominantly found in thalamic (75%) and cerebral hemispheric tumors (62.2%) [7]. Zhang et al. and Louis et al. reported Tp53 mutations in 61.5% and 71% of their cases, respectively [17, 18].
Schwartzentruber et al. have reported mutations and loss of ATRX expression in one-third (33%) of pediatric GBM. The location-wise distribution was not elaborated; however, most of the tumors in this series were either hemispheric or thalamic in location [19]. Our series showed much higher (69%) incidence of loss of ATRX expression in pGBM. Khuong-Quang et al. reported that ATRX mutation was common in diffuse intrinsic pontine glioma (DIPG) and in older children [20]. Our series demonstrated ATRX loss in 84% of hemispheric tumors, 66% of thalamic tumors, and 42.8% of brain stem tumors. Data regarding the incidence of IDH mutations in pediatric GBM is sparse. We noted IDH1 (R132H) immunopositivity in 6.9% of cases, and this was found only in hemispheric tumors. In concurrence with the existing literature, we identified that 14 out of 29 tumors showed H3K27M immunopositivity and these were mostly of midline location (13/14; 92.8%). Khuong-Quang et al. also reported that H3K27M-mutated pGBM usually are associated with deep-seated lesions, close to midline (thalamus and brain stem) location, and carry a poorer prognosis [20]. Similar results were shown by Bjerke et al. [21].
The CCG-945 trial demonstrated that mutations in the TP53 gene had low progression-free survival (PFS). Similarly, Pollack et al. have reported a 5-year PFS of patients with low levels of p53 expression was significantly higher than that in children with an overexpression of p53 [22]. In our study, the P53-negative tumors had a better survival when compared with the P53-positive tumors, though not statistically significant.
We noted that tumors which showed loss of ATRX expression had longer overall survival compared with those with retained expression (p = 0.093; trend to significance). It is likely that ATRX mutation may be associated with a better prognosis in pGBM. However, the survival analysis for ATRX expression in pGBMs has not been outlined by any study previously, to the best of our knowledge. We also noted that tumors with H3K27M mutations had significantly worse prognosis, in concordance with the data reported by other authors [19, 20]. Khuong et al., in their series on pGBM, reported that the mean overall survival for patients with K27M-H3.3-mutated tumors was 0.73 years (± 0.48) versus 4.59 years (± 5.55) (p = 0.0008) for patients with wild-type tumors. Korshunov et al., in their series on pediatric GBM, have reported that any oncogene amplification and/or K27M mutation (n = 124) represents a particularly unfavorable group, with a 3-year overall survival (OS) of 5%, whereas tumors without these markers (n = 38) define a more favorable group (3-year OS ~ 70%) [23].
While H3K27M mutations are found exclusively in the diffuse midline gliomas and are associated with a worse clinical outcome, the cerebral hemispheric GBMs often harbor H3.3G34R (or rarely G34V) mutations and this is associated with a favorable outcome compared with tumors carrying the K27M mutation [19, 24, 25]. However, in this study, we were not able to assess the latter. Other mutations such as Tp53 and ATRX have been described in both cerebral hemispheric and brain stem GBMs.
In a meta-analysis of 1067 cases of pediatric high-grade glioma and DIPG, Mackay et al. reported the median overall survival for midline HGG to be 13 months and DIPG to be 10.8 months. Out of 903 cases for which hotspot mutation data for genes encoding histone H3 were available, 316 cases were found to be positive for H3.3K27M mutation. These H3.3K27M mutations were spread throughout the midline and pons, where they accounted for 63.0% DIPG and 59.7% non-brain stem midline tumors. On multivariate analysis, including the histone mutations, age, WHO grade, and gender, they noted that K27M mutations in both H3.3 and H3.1 were independently associated with a shorter survival (p < 0.0001) [26].
Korshunov et al. using integrated genomic and epigenomic platform have identified four molecular subgroups of pGBM with each of these subgroups differing in their clinical outcome [23]. In the present study, by IHC technique, we were able to reiterate the fact that the molecular mechanisms involved in the development of hemispheric and midline tumors appear to be different in pediatric GBM and also play an important role on outcome of patients.
Conclusions
Our study is one among the few studies from India evaluating the clinical and immunohistochemical profile in pGBM and their prognostic significance. Preoperative KPS, extent of surgical resection, adjuvant radiotherapy, location of tumors, and H3K27M immunopositivity were found to be the factors influencing median survival on univariate analysis.
References
Song KS, Phi JH, Cho BK, Wang KC, Lee JY, Kim DG, Kim IH, Ahn HS, Park SH, Kim SK (2010) Long-term outcomes in children with glioblastoma. J Neurosurg Pediatr 6:145–149
Perkins SM, Rubin JB, Leonard JR, Smyth MD, El Naqa I, Michalski JM, Simpson JR, Limbrick DL, Park TS, Mansur DB (2011) Glioblastoma in children: a single-institution experience. Int J Radiat Oncol Biol Phys 80:1117–1121
Das KK, Mehrotra A, Nair AP, Kumar S, Srivastava AK, Sahu RN, Kumar R (2012) Pediatric glioblastoma: clinico-radiological profile and factors affecting the outcome. Childs Nerv Syst 28:2055–2062
Suri V, Das P, Pathak P, Jain A, Sharma MC, Borkar SA, Suri A, Gupta D, Sarkar C (2009) Pediatric glioblastomas: a histopathological and molecular genetic study. Neuro-oncology 11:274–280
Jaiswal Janhvi SAH, Arvind R, Chickabasaviah Yasha T, Arivazhagan A, Vani S (2016) Spectrum of primary intracranial tumors at a tertiary care neurological institute: a hospital-based brain tumor registry. Neurol India 64:494–501
Diaz AK, Baker SJ (2014) The genetic signatures of pediatric high-grade glioma: no longer a one-act play. Semin Radiat Oncol 24:240–247
Ganigi PM, Santosh V, Anandh B, Chandramouli BA, Sastry Kolluri VR (2005) Expression of p53, EGFR, pRb and bcl-2 proteins in pediatric glioblastoma multiforme: a study of 54 patients. Pediatr Neurosurg 41:292–299
Adams H, Adams HH, Jackson C, Rincon-Torroella J, Jallo GI, Quinones-Hinojosa A (2016) Evaluating extent of resection in pediatric glioblastoma: a multiple propensity score-adjusted population-based analysis. Childs Nerv Syst 32:493–503
Manoj N, Arivazhagan A, Bhat DI, Arvinda HR, Mahadevan A, Santosh V, Devi BI, Sampath S, Chandramouli BA (2014) Stereotactic biopsy of brainstem lesions: techniques, efficacy, safety, and disease variation between adults and children: a single institutional series and review. J Neurosci Rural Pract 5:32–39
Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D (1989) The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neuro-Oncol 7:165–177
Nikitović M, Stanić D, Pekmezović T, Gazibara MS, Bokun J, Paripović L, Grujičić D, Sarić M, Mišković I (2016) Pediatric glioblastoma: a single institution experience. Childs Nerv Syst 32:97–103
Artico M, Cervoni L, Celli P, Salvati M, Palma L (1993) Supratentorial glioblastoma in children: a series of 27 surgically treated cases. Childs Nerv Syst 9:7–9
Shinojima N, Kochi M, Hamada J, Nakamura H, Yano S, Makino K, Tsuiki H, Tada K, Kuratsu J, Ishimaru Y, Ushio Y (2004) The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme. J Neurosurg 101:219–226
Bhattacharjee MB, Bruner JM (1997) p53 protein in pediatric malignant astrocytomas: a study of 21 patients. J Neuro-Oncol 32:225–233
Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW (2000) Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol 10:249–259
Sure U, Ruedi D, Tachibana O, Yonekawa Y, Ohgaki H, Kleihues P, Hegi ME (1997) Determination of p53 mutations, EGFR overexpression, and loss of p16 expression in pediatric glioblastomas. J Neuropathol Exp Neurol 56:782–789
Louis DN, Rubio MP, Correa KM, Gusella JF, von Deimling A (1993) Molecular genetics of pediatric brain stem gliomas. Application of PCR techniques to small and archival brain tumor specimens. J Neuropathol Exp Neurol 52:507–515
Zhang SJ, Feng XL, Koga H, Ichikawa T, Abe S, Kumanishi T (1993) p53 gene mutations in pontine gliomas of juvenile onset. Biochem Biophys Res Commun 196:851–857
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231
Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, Bartels U, Albrecht S, Schwartzentruber J, Letourneau L, Bourgey M, Bourque G, Montpetit A, Bourret G, Lepage P, Fleming A, Lichter P, Kool M, von Deimling A, Sturm D, Korshunov A, Faury D, Jones DT, Majewski J, Pfister SM, Jabado N, Hawkins C (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124:439–447
Bjerke L, Mackay A, Nandhabalan M, Burford A, Jury A, Popov S, Bax DA, Carvalho D, Taylor KR, Vinci M, Bajrami I, McGonnell IM, Lord CJ, Reis RM, Hargrave D, Ashworth A, Workman P, Jones C (2013) Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discov 3:512–519
Pollack IF, Finkelstein SD, Woods J, Burnham J, Holmes EJ, Hamilton RL, Yates AJ, Boyett JM, Finlay JL, Sposto R (2002) Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med 346:420–427
Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D, Meyer J, Schrimpf D, Kool M, Northcott PA, Zheludkova O, Milde T, Witt O, Kulozik AE, Reifenberger G, Jabado N, Perry A, Lichter P, von Deimling A, Pfister SM, Jones DT (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129:669–678
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C, Pfaff E, Tonjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu XY, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Fruhwald MC, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Durken M, Kulozik AE, Madden J, Donson A, Foreman NK, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm CM, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth AM, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski PP, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor MD, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister SM (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Fontebasso AM, Liu XY, Sturm D, Jabado N (2013) Chromatin remodeling defects in pediatric and young adult glioblastoma: a tale of a variant histone 3 tail. Brain Pathol 23:210–216
Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, Bjerke L, Clarke M, Vinci M, Nandhabalan M, Temelso S, Popov S, Molinari V, Raman P, Waanders AJ, Han HJ, Gupta S, Marshall L, Zacharoulis S, Vaidya S, Mandeville HC, Bridges LR, Martin AJ, Al-Sarraj S, Chandler C, Ng HK, Li X, Mu K, Trabelsi S, Brahim DH, Kisljakov AN, Konovalov DM, Moore AS, Carcaboso AM, Sunol M, de Torres C, Cruz O, Mora J, Shats LI, Stavale JN, Bidinotto LT, Reis RM, Entz-Werle N, Farrell M, Cryan J, Crimmins D, Caird J, Pears J, Monje M, Debily MA, Castel D, Grill J, Hawkins C, Nikbakht H, Jabado N, Baker SJ, Pfister SM, Jones DTW, Fouladi M, von Bueren AO, Baudis M, Resnick A, Jones C (2017) Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 32:520–537 e525
Acknowledgments
We would like to acknowledge Mr. MR Chandrashekar and Mrs. Hemavathy U for technical assistance and Mr. Manjunath K for the preparation of the picture montages.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study has been approved by the Institute Ethics committee, NIMHANS, Bangalore.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uppar, A.M., Sugur, H., Prabhuraj, A.R. et al. H3K27M, IDH1, and ATRX expression in pediatric GBM and their clinical and prognostic significance. Childs Nerv Syst 35, 1537–1545 (2019). https://doi.org/10.1007/s00381-019-04222-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04222-z