Abstract
Metastasis of an intracranial tumour is not a common situation in our daily neurosurgical practice. Pleomorphic xanthoastrocytoma is also a rare glial tumour with relatively a favourable prognosis among other CNS pathologies. Here, we present an anaplastic pleomorphic xanthoastrocytoma case which shows both haematogenous and lymphatic metastasis which is described first time in the up-to-date literature. Our case is a 17-year-old male operated for a right occipital intra-axial lesion with a diagnosis of anaplastic pleomorphic xanthoastrocytoma which recurs 5 years later and metastasize vigorously through haematogenous and lymphatic routes. A rare-presenting symptom for this pathology is also intracerebral haemorrhage. This is the ninth case report in the literature which presents initially with this entity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pleomorphic xanthoastrocytoma (PXA) is an uncommon (< 1%) central nervous system tumour most commonly seen in children and young adults that undergo anaplastic transformation in 15 to 20% of cases [1]. Kepes et al. [2] first described it in 1979 as a type of distinct astrocytic tumour. Most of these tumours are located in the supratentorial area, mainly in the temporal lobes. Therefore, the most common initial-presenting symptom is seizures [3]. The prognosis is favourable for this tumour, with a 30% recurrence rate in 5 years and 40% in 10 years following gross total resection and an overall survival rate of 80% and 70% in 5 and 10 years, respectively [2, 4]. Intracerebral haemorrhage is also a rare-presenting symptom for this pathology. PXA with anaplastic features, which display increased mitotic activity with or without accompanying necrosis, is defined as grade III tumours according to WHO classification [5].
To date, there has been only one case report that shows scalp and sacral metastasis via a haematogenous route [6], and here, we present the first case in the literature of a PXA that has metastasized via lymphatic and haematogenous ways to the mediastinum and extracranial skeleton. This case is the ninth reported case in the literature with intracerebral haemorrhage as an initial-presenting symptom.
Case
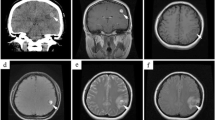
The seventeen-year-old male patient was admitted to our clinic with a complaint of headache with no neurologic deficit that had been present for the previous 2 years. Cranial magnetic resonance imaging (MRI) showed that he had a right occipital intra-axial lesion that was 2.5 × 3 cm in diameter after diffuse enhancement with an IV gadolinium injection. The lesion had a 6- to 7-mm haemorrhagic component in the central portion. MR spectroscopic investigation was consistent with a glial tumour (Fig. 1), and a gross total resection was performed via a right occipital craniotomy.
Under haematoxylin and eosin staining (Fig. 2a), the tumour cells exhibited classic features with pleomorphic and xanthomatous cells. Frequent multinucleated and enlarged cells with giant, oddly-shaped nuclei with occasional nuclear inclusion were seen. Brisk mitotic activity and necrosis were identified, and perivascular lymphocytic cuffing and scattered eosinophilic granular bodies were also seen. A reticulin rich network was identified throughout the tumour under reticulin staining (Fig. 2b). Immunohistochemically, both glial fibrillary acidic protein (GFAP) (Fig. 2c) and synaptophysin expressions in the large pleomorphic and xanthomatous cells revealed the biphenotypic glioneuronal appearance of the tumour cells. The Ki67 proliferation index was 30% (Fig. 2d). The final diagnosis was consistent with anaplastic pleomorphic xanthoastrocytoma (PXA) (WHO 2016) [5] infiltrating the dura. The patient received 5940-cGy adjuvant radiotherapy by the intensity-modulated radiation therapy (IMRT) method. He was seen under follow-up with no complaint and no radiologic progression.
a H + E, X400, bizarre giant cells and a smaller population of tumour cells with occasional cytoplasmic lipidization. b Reticulin, X400, pericellular reticulogenesis. c GFAP, X400, cytoplasmic positivity of glial fibrillary acidic protein in tumour cells. d Ki67, X400, nuclear immunoreactivity for Ki67 in tumour cells associated with an atypical mitosis at the upper left (arrow)
Five years later, he was admitted to hospital with sudden onset of headache and loss of consciousness. Cranial computerised tomography (CT) investigation showed a left frontal acute intracerebral hematoma of 5 cm in diameter (Fig. 3a, b). The patient had a cranial MR (Fig. 3c, d), 4D dynamic MR angiography and conventional cerebral angiography investigations. A capillary haemangioma was present in the central portion of the hematoma with no other specific features. The patient then underwent a left frontal craniotomy and hematoma evacuation. A postoperative CT investigation showed no residual hematoma and no additional neurologic deficit.
During routine preoperative workups, a PA chest x-ray showed that bilateral intraparenchymal and hilar lesions had appeared. A thorax CT was performed, and bilateral hilar and mediastinal lesions were seen, with the lesion on the right side having a maximum diameter of 9.5 cm and the one on the left side having a diameter of 6.5 cm at its widest (Fig. 4a). Bilateral intraparenchymal nodules were present, and the largest of these had a diameter of 2.2 cm; this was consistent with the metastatic appearance of the left side. The patient underwent a mediastinal lymph node biopsy via mediastinoscopy. Histopathological examination showed a metastatic astrocytic tumour invading one of the two lymph nodes. The histomorphological features of this material and the initial tumour tissue, as taken in the first craniotomy, were similar, so this recurrence was considered to be metastatic anaplastic PXA. The tumour was composed of oval and fusiform astrocytic-like cells, and while these oval cells had hyperchromatic nuclei with ample cytoplasm, the fusiform cells had bipolar nuclei and cytoplasm. Pleomorphism and mitosis were present, though necrosis was not seen. Immunohistochemical study showed positive results for GFAP, olig2, synaptophysin and VEGF. IDH, NeuN, NFP, p53 and EGFR were negative in the tumour cells. MGMT was positive in 40 to 60% of tumour cells. The pathological samples which were taken from the hematoma border were also consistent with anaplastic PXA, with a Ki67 proliferative index of 30%.
A whole body PET–CT was performed to grade the disease; during this, mediastinal and intraparenchymal lung lesions above a new right frontal, C1 left arcus, right iliac bone and left ischium settled hyper metabolic lesions were seen (Fig. 4b, c, d). A cranial MR investigation using an IV gadolinium injection was performed, and a right frontal lesion was reported that was identified as a hematoma. One month later, this MR investigation was repeated, and a small resorption of the hematoma was seen.
The patient consulted with the medical oncology department, and systemic chemotherapy (zoledronic acid and temozolomide) was started. After two cycles of chemotherapy, a cranial MR investigation was performed again, and the right frontal hematoma had progressed, showing increased contrast enhancement and perilesional oedema with midline shift (Fig. 5a, b). MR spectroscopic evaluation could not be performed due to the increased blood component in the lesion [7]. Additionally, at the first operation site, ring contrast enhancement was seen, which was reported as tumour progression. The patient had a right frontal craniotomy, and the pathology was again consistent with anaplastic PXA (WHO 2016) [5].
Two months after his third craniotomy, the patient underwent a control MRI that showed multicentric contrast-enhancing lesions. The lesions were accepted as high-grade glioma, and he was referred to whole brain radiation therapy. Despite WBRT and chemotherapy, the patient died due to tumour progression 6 months after the final operation. His overall survival time was 72 months.
Discussion
The WHO defines PXA as a rarely seen (< 1%) grade III tumour [5]. Gross total resection of the tumour constitutes the majority of treatment [8], and radiologic follow-up and re-surgery are recommended if recurrence is detected. Anaplastic PXA was first described in 1999 [9] as having high mitotic activity with or without accompanying necrosis. To date, 55 PXA patients with anaplastic features have been presented in the literature as an initial diagnosis [4, 10,11,12,13,14,15,16,17]. Thirty-one PXA patients with malignant transformation (to anaplastic PXA or high-grade glial tumours) have been featured in the literature (Table 1).
Spontaneous intratumoural bleeding as seen in our case is rarely seen in PXA patients as an initial-presenting symptom. There have been only eight cases presented with haemorrhage in the literature so far [17,18,19,20,21,22,23,24] (Table 2). This has been linked to meningeal involvement in one of the cases [23], and a pseudoaneurysm formation due to vascular invasion by the tumour was seen in another case [18]; however, the exact mechanism is still unclear in our case and several others.
To date, there is only one case reported in the literature showing haematogenous metastasis to the sacrum and lumbar vertebrae with scalp metastasis 4 years from initial diagnosis; this was seen in a 27-year-old man [6]. The first diagnosis was PXA, and after a second recurrence, it transformed into anaplastic oligodendroglioma. The patient had repeated surgeries and radiotherapy but was lost in follow-up. Overall survival could not therefore be observed. Our case is the first one in the literature showing both haematogenous and lymphatic metastasis, as proved by lymph nodule biopsy of the mediastinum and whole body PET–CT investigation.
There is no approved treatment protocol for PXA with anaplastic features. According to the literature, adjuvant radiotherapy [6, 14, 25,26,27] is applied to those patients who have anaplastic PXA as an initial diagnosis. The tumour board of our institution therefore recommended radiation treatment in our case.
Adjuvant chemotherapy with temozolomide, which is an alkylating agent used in glioblastoma patients, has been tried in some cases [25, 27]. When using temozolomide, MGMT methylation status is an important prognostic factor of better response to treatment with better outcomes [28]. Marucci et al. [16] looked at the MGMT methylation status of 11 PXA patients, nine of whom had a diagnosis of PXA, and two of whom had a diagnosis of PXA with anaplastic features; this showed that only two PXA patients had a methylated MGMT gene. Neither of these patients recurred, but one patient with PXA with anaplastic features had recurred 3 years later, and the other was in follow-up when the paper was submitted. We tried temozolomide in our case after the second craniotomy, along with zoledronic acid to address the patient’s bone metastasis. Before chemotherapy started, a right frontal lesion was detected and initially reported as a haematoma; this had regressed 1 month later. Chemotherapy was started, but after two cycles, the lesion progressed and a third surgery was performed. The patient’s MGMT status was methylated, but despite this favourable condition, chemotherapy was ineffective for the intracranial portion.
Conclusion
PXA is a rare low-grade astrocytoma with a generally favourable prognosis. However, anaplastic PXA shows more aggressive behaviours in terms of recurrence intervals and ability to metastasize. Therefore, PXA with anaplastic features requires more aggressive or more specifically targeted therapy; nevertheless, despite such aggressive therapy, this type of tumour can metastasize in both lymphatic and haematogenous ways, and may present with intracerebral haemorrhage.
References
Perkins SM, Mitra N, Fei W, Shinohara ET (2012) Patterns of care and outcomes of patients with pleomorphic xanthoastrocytoma: a SEER analysis. J Neuro-Oncol 110:99–104
Kepes JJ, Rubinstein LJ, Eng LF (1979) Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer 44:1839–1852
Ozek MM, Sav A, Pamir MN, Ozer AF, Ozek E, Erzen C (1993) Pleomorphic xanthoastrocytoma associated with von Recklinghausen neurofibromatosis. Child’s Nervous System : ChNS : Official J Int Soc Pediatric Neurosurgery 9:39–42
Rao AA, Laack NN, Giannini C, Wetmore C (2010) Pleomorphic xanthoastrocytoma in children and adolescents. Pediatr Blood Cancer 55:290–294
David N. Louis HO, Otmar D. Wiestler, Webster K. Cavenee (2016) WHO classification of tumours of the central nervous system (Revised 4th edition) International Agency for Research On Cancer, Lyon
Foo J, Ng WH (2011) Metastatic pleomorphic xanthoastrocytoma in the scalp. J Clinical Neuroscience : Official J Neurosurgical Soc Australasia 18:565–567
Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dincer A, Dydak U, Emir UE, Frahm J, Gonzalez RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Huppi PS, Hurd RE, Kantarci K, Klomp DW, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjanska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJ, Ratai EM, Ross BD, Scheenen TW, Schuster C, Smith IC, Soher BJ, Tkac I, Vigneron DB, Kauppinen RA, Group MRSC (2014) Clinical proton MR spectroscopy in central nervous system disorders. Radiology 270:658–679
Fouladi M, Jenkins J, Burger P, Langston J, Merchant T, Heideman R, Thompson S, Sanford A, Kun L, Gajjar A (2001) Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection. Neuro-Oncology 3:184–192
Giannini C, Scheithauer BW, Burger PC, Brat DJ, Wollan PC, Lach B, O’Neill BP (1999) Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer 85:2033–2045
Gaskill SJ, Marlin AE, Saldivar V (1988) Glioblastoma multiforme masquerading as a pleomorphic xanthoastrocytoma. Child’s nervous system : ChNS : official J Int Soc Pediatric Neurosurgery 4:237–240
Tonn JC, Paulus W, Warmuth-Metz M, Schachenmayr W, Sorensen N, Roosen K (1997) Pleomorphic xanthoastrocytoma: report of six cases with special consideration of diagnostic and therapeutic pitfalls. Surg Neurol 47:162–169
Chakrabarty A, Mitchell P, Bridges LR, Franks AJ (1999) Malignant transformation in pleomorphic xanthoastrocytoma--a report of two cases. Br J Neurosurg 13:516–519
Marton E, Feletti A, Orvieto E, Longatti P (2007) Malignant progression in pleomorphic xanthoastrocytoma: personal experience and review of the literature. J Neurol Sci 252:144–153
Benjamin C, Faustin A, Snuderl M, Pacione D (2015) Anaplastic pleomorphic xanthoastrocytoma with spinal leptomeningeal spread at the time of diagnosis in an adult. J Clinical Neuroscience : Official J Neurosurgical Soc Australasia 22:1370–1373
Gallo P, Cecchi PC, Locatelli F, Rizzo P, Ghimenton C, Gerosa M, Pinna G (2013) Pleomorphic xanthoastrocytoma: long-term results of surgical treatment and analysis of prognostic factors. Br J Neurosurg 27:759–764
Marucci G, Morandi L (2011) Assessment of MGMT promoter methylation status in pleomorphic xanthoastrocytoma. J Neuro-Oncol 105:397–400
Asano K, Miyamoto S, Kubo O, Kikkukawa T, Yagihashi A, Ohkuma H (2006) A case of anaplastic pleomorphic xanthoastrocytoma presenting with tumor bleeding and cerebrospinal fluid dissemination. Brain Tumor Pathology 23:55–63
Yoshikawa G, Kawamoto S, Yakou K, Tsutsumi K (2010) Massive intracranial hemorrhage associated with pleomorphic xanthoastrocytoma--case report. Neurol Med Chir 50:220–223
Wind JJ, Kerr PB, Sweet JA, Deshmukh VR (2009) Pleomorphic xanthoastrocytoma presenting with life-threatening hemorrhage in a child. J Neurosurg Pediatr 3:157–159
Lee DK, Cho KT, Im SH, Hong SK (2007) Pleomorphic xanthoastrocytoma with an intracystic hemorrhage : a case report and literature review. J Korean Neurosurgical Soc 42:410–412
Abe T, Inoue R, Isono M, Ishii K, Fujiki M, Kamida T, Kobayashi H, Kashima K, Kusakabe T, Nakazato Y (2006) Benign pleomorphic astrocytoma in the hypothalamus--case report. Neurol Med Chir 46:101–103
Yoshida D, Kogiku M, Noha M, Takahashi H, Teramoto A (2005) A case of pleomorphic xanthoastrocytoma presenting with massive tumoral hemorrhage. J Neuro-Oncol 71:169–171
Levy RA, Allen R, McKeever P (1996) Pleomorphic xanthoastrocytoma presenting with massive intracranial hemorrhage. AJNR Am J Neuroradiol 17:154–156
Lim S, Kim JH, Kim SA, Park ES, Ra YS, Kim CJ (2013) Prognostic factors and therapeutic outcomes in 22 patients with pleomorphic xanthoastrocytoma. J Korean Neurosurgical Soc 53:281–287
Binesh F, Akhavan A, Navabii H (2012) Pleomorphic xanthoastrocytoma with malignant transformation and multiple recurrences in an Iranian girl. BMJ Case Reports 2012:bcr1220115372
Vu TM, Liubinas SV, Gonzales M, Drummond KJ (2012) Malignant potential of pleomorphic xanthoastrocytoma. J Clinical Neuroscience : Official J Neurosurgical Soc Australasia 19:12–20
Alexiou GA, Moschovi M, Stefanaki K, Prodromou C, Sfakianos G, Prodromou N (2010) Malignant progression of a pleomorphic xanthoastrocytoma in a child. Neuropediatrics 41:69–71
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Weldon-Linne CM, Victor TA, Groothuis DR, Vick NA (1983) Pleomorphic xanthoastrocytoma. Ultrastructural and immunohistochemical study of a case with a rapidly fatal outcome following surgery. Cancer 52:2055–2063
Kepes JJ, Rubinstein LJ, Ansbacher L, Schreiber DJ (1989) Histopathological features of recurrent pleomorphic xanthoastrocytomas: further corroboration of the glial nature of this neoplasm. A study of 3 cases. Acta Neuropathologica 78:585–593
Allegranza A, Ferraresi S, Bruzzone M, Giombini S (1991) Cerebromeningeal pleomorphic xanthoastrocytoma. Report on four cases: clinical, radiologic and pathological features. (including a case with malignant evolution). Neurosurg Rev 14:43–49
Macaulay RJ, Jay V, Hoffman HJ, Becker LE (1993) Increased mitotic activity as a negative prognostic indicator in pleomorphic xanthoastrocytoma. Case report. J Neurosurgery 79:761–768
van Roost D, Kristof R, Zentner J, Wolf HK, Schramm J (1996) Clinical, radiological, and therapeutic features of pleomorphic xanthoastrocytoma: report of three patients and review of the literature. J Neurol Neurosurg Psychiatry 60:690–692
Bayindir C, Balak N, Karasu A, Kasaroglu D (1997) Anaplastic pleomorphic xanthoastrocytoma. Child’s Nervous System : ChNS : Official J Int Soc Pediatric Neurosurgery 13:50–56
Charbel FT (1998) Pleomorphic xanthoastrocytoma with malignant progression. Surg Neurol 50:385–386
Leonard N, Alcutt DA, Farrell MA (1998) Fatal pleomorphic xanthoastrocytoma with meningeal gliomatosis. Histopathology 32:375–378
Prayson RA, Morris HH 3rd (1998) Anaplastic pleomorphic xanthoastrocytoma. Archives Pathology Laboratory Medicine 122:1082–1086
de Tella OI Jr, Herculano MA, Prandini MN, Stavale JN, Aguiar PH (2003) Malignant transformation of pleomorphic xanthoastrocytoma: case report. Arq Neuropsiquiatr 61:104–106
Klein O, Grignon Y, Pinelli C, Civit T, Auque J, Marchal JC (2004) Pleomorphic xanthoastrocytoma. A review of five observations. Neuro-Chirurgie 50:515–520
Tan TC, Ho LC, Yu CP, Cheung FC (2004) Pleomorphic xanthoastrocytoma: report of two cases and review of the prognostic factors. J Clinical Neuroscience : official J Neurosurgical Soc Australasia 11:203–207
Saikali S, Le Strat A, Heckly A, Stock N, Scarabin JM, Hamlat A (2005) Multicentric pleomorphic xanthoastrocytoma in a patient with neurofibromatosis type 1. Case report and review of the literature. J Neurosurg 102:376–381
Nakajima T, Kumabe T, Shamoto H, Watanabe M, Suzuki H, Tominaga T (2006) Malignant transformation of pleomorphic xanthoastrocytoma. Acta Neurochir 148:67–71 discussion 71
Rodriguez-Mena R, Joanes-Alepuz V, Barbella-Aponte R, Perez-Valles A (2012) Pleomorphic xanthoastrocytoma with intraventricular extension and anaplastic transformation in an adult patient: case report. Neurocirugia 23:203–210
Frank S, Cordier D, Tolnay M, Rosenblum MK (2009) A 28-year-old man with headache, visual and aphasic speech disturbances. Brain Pathology 19:163–166
Harada S, Fallon KB, Reddy A, Nabors LB (2014) Molecular pathology: SC18-1 interesting case - actionable mutation in a case with a recurrent pleomorphic xanthoastrocytoma with anaplastic features. Pathology 46(Suppl 2):S28–S29
Tanaka S, Nakada M, Nobusawa S, Suzuki SO, Sabit H, Miyashita K, Hayashi Y (2014) Epithelioid glioblastoma arising from pleomorphic xanthoastrocytoma with the BRAF V600E mutation. Brain Tumor Pathology 31:172–176
Acknowledgments
The authors thank Mrs. Özlem Yapıcıer, MD for her contribution for editing figures and details related to pathological headings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Demirci Otluoğlu, G., Özek, M.M. A rare clinical presentation: a pleomorphic xanthoastrocytoma presenting with intracerebral haemorrhage and metastasizing vigorously—case report and review of the literature. Childs Nerv Syst 35, 355–362 (2019). https://doi.org/10.1007/s00381-018-3960-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3960-1