Abstract
Purpose
Increased serum biomakers, such as S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE), are associated with traumatic brain injury (TBI). The purpose of this study is to investigate the serum levels of S100B and NSE in pediatric TBI patients and to predict a clinical outcome.
Methods
Peripheral venous blood was collected within 6 h of injury and at 1 week to measure S100B and NSE. The serum S100B and NSE levels were measured using commercially available enzyme-linked immunosorbent assay kits. The authors divided participants into two groups at admission: a favorable group (patients with Glasgow Coma Scale [GCS] scores of 10–15) and an unfavorable group (patients with GCS scores of less than 9). Both S100B and NSE levels were compared between the two groups at the time of admission and 1 week later.
Results
Ten pediatric patients were enrolled (5 in the favorable group, 5 in the unfavorable group). The median serum S100B level of 134.21 pg/ml (range, 51.00–789.65 pg/ml) in patients with TBI at admission dropped to 41.49 pg/ml (range, 25.65–260.93 pg/ml) after 1 week, with significant differences between the traumatic event and 1 week later (p = 0.007). The median serum NSE level of 14.76 ng/ml (range, 6.48–21.23 ng/ml) in patients with TBI at admission was higher than that after 1 week (4.96 ng/ml, range, 3.01–31.21 ng/ml), with significant differences (p = 0.015). A significant difference was observed in S100B after 1 week between patients in the favorable and unfavorable groups (p = 0.047). One patient whose serum S100B and NSE levels were elevated 1 week after TBI eventually died.
Conclusions
Elevated serum S100B and NSE levels in pediatric TBI patients decreased 1 week after traumatic events. The serum S100B level 1 week after TBI was related to the severity of brain damage. These results indicated that serum S100B and NSE might play a role in predicting the prognosis and monitoring ongoing brain injury in pediatric TBI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pediatric traumatic brain injury (TBI) is a common cause of death and can often leave serious sequela. The evaluation of TBI in children is not easy. It is difficult to evaluate pediatric patients with repeated brain computed tomography (CT) scans or invasive intracranial pressure (ICP) monitors. Non-invasive, repetitive, and simple serum biomarkers measurement can be especially helpful for children if the severity of brain damage can be determined and the clinical outcome can be predicted.
Over the past years, serum biomarkers such as S100 calcium-binding protein B (S100B) and neuron-specific enolase (NSE) have been introduced to diagnose brain injury [1,2,3]. S100B is mainly released by glial cells and has a serum half-life of 60–120 min. NSE is primarily located in the cytoplasm of neurons and is the only marker that assesses damage to neurons. It has a serum half-life of > 20 h. It is assumed that after TBI, the membrane integrity of the brain cells is disturbed and S100B and NSE are released into the blood [4].
In this study, the serum S100B and NSE levels in children with TBI were evaluated at admission and 1 week after trauma. We hypothesized that we could predict the clinical outcome and monitor ongoing brain injury from the serum S100B and NSE levels.
Clinical material and methods
Patient population
Ten pediatric patients with TBI who provided written informed consent were enrolled in this study. The prospective protocol was reviewed and approved by the Institutional Review Board of our hospital. All patients enrolled in the study arrived at the hospital within 6 h after head trauma. Children with concomitant injuries were included. To assess injury severity, we divided the patients into two groups at admission: a favorable group (patients with Glasgow Coma Scale [GCS] scores of 10–15) and an unfavorable group (patients with GCS scores of less than 9). To evaluate the clinical outcome, we used Glasgow Outcome Scale (GOS) 6 months after TBI.
Sample collection and enzyme-linked immunosorbent assays (ELISAs) for S100B and NSE
To measure the serum S100B and NSE levels, peripheral venous whole blood was obtained at admission and 1 week after TBI. Blood samples from the study participants were collected in citrate-supplemented tubes and centrifuged at 3200 rpm for 10 min at 4 °C. The supernatant was transferred to a microtube and frozen immediately at − 80 °C until analysis. The analysis was performed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. All patient samples collected were assayed simultaneously.
Statistical analysis
We analyzed the serum S100B and NSE levels at admission and 1 week after TBI and analyzed the correlation of S100B and NSE. We evaluated differences in the serum biomarkers level between the favorable and unfavorable groups at admission. All analyses were performed using SPSS 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). Because the data were not distributed normally, they were analyzed using the Wilcoxon rank-sum test and the Mann-Whitney U test. Correlations were assessed using the nonparametric Spearman’s rank correlation analysis. p values < 0.05 were considered significant.
Results
Of the ten consecutive patients enrolled, eight were men and two were women (median age, 15 years; age range, 6–18 years). The patients had various types of traumatic brain hemorrhage: epidural hemorrhage (6 patients), contusion (2 patients), diffuse axonal injury (1 patient), and subarachnoid hemorrhage (1 patient). Peripheral venous blood was sampled from all patients at admission and 1 week after TBI. The patient characteristics are summarized in Table 1. Mental change (60%) and headache (40%) were the most common symptoms after TBI. Of the six mentally deteriorated patients, four were stuporous, one was drowsy, and one was semi-comatose. The GCS at admission was 15 in 2 patients, 14 in 3 patients, 9 in 4 patients, and 4 in 1 patient. The GOS at 6 months was 5 (good recovery) in 7 patients, 4 (moderate disability but independent) in 2 patients, and 1 (death) in 1 patient. Four patients underwent craniotomy or craniectomy for removal of hematoma. Three patients experienced concomitant trauma including the lung, bone, liver, and bowel.
Serum S100B and NSE concentrations after TBI
Median serum S100B and NSE concentrations at admission after TBI were 134.21 pg/ml and 14.76 ng/ml, respectively (Table 2). No significant differences were observed in the serum S100B and NSE levels between men and women (p = 0.400 and 0.711, respectively). No correlation was found between the serum concentrations of the S100B and NSE levels and age (p = 0.067 and 0.370, respectively). The various types of traumatic brain hemorrhage did not significantly affect the serum levels of S100B (p = 0.565) and NSE (p = 0.647). No significant difference was shown between the serum S100B and NSE levels and the presence of concomitant injury (p = 0.809 and 0.809, respectively). The difference between surgical and non-surgical treatment for TBI had no significant effect on the serum levels of S100B (p = 0.286) and NSE (p = 0.394).
The correlation between the serum S100B and NSE levels at admission was positive (p = 0.004, r = 0.818). Moreover, the serum S100B and NSE levels 1 week after trauma showed a significant positive correlation (p = 0.001, r = 0.952). The twice measured serum S100B and NSE levels also showed a positive relation (p = 0.001, r = 0.898) (Fig. 1).
The median serum S100B level of 134.21 pg/ml (range, 51.00–789.65 pg/ml) in patients with TBI at admission dropped to 41.49 pg/ml (range, 25.65–260.93 pg/ml) after 1 week, with significant differences between measurements within 6 h after the traumatic event and 1 week later (p = 0.007) (Fig. 2). The median serum NSE level of 14.76 ng/ml (range, 6.48–21.23 ng/ml) in patients with TBI at admission was higher than that after 1 week (4.96 ng/ml, range, 3.01–31.21 ng/ml), with significant differences (p = 0.015) (Fig. 3).
The median serum S100B level (52.15 pg/ml) 1 week after TBI in the unfavorable group was higher than that in the favorable group (29.33 pg/ml), with significant differences (p = 0.047) (Fig. 4). The median serum NSE level at admission (18.02 ng/ml), the NSE level 1 week after trauma (6.65 ng/ml), and the S100B level at admission (178.12 pg/ml) in the unfavorable group were higher than those in the favorable group (6.92 ng/ml, 3.22 ng/ml, and 77.26 pg/ml, respectively). However, no statistical differences were found in the favorable and unfavorable groups (p = 0.076, 0.076, and 0.251, respectively). In the unfavorable group, one patient diagnosed with diffuse axonal injury was semi-comatose. In the patient, the serum S100B level of 178.12 pg/ml and the NSE level of 14.29 ng/ml at admission were elevated to 260.93 pg/ml (serum S100B level) and 31.21 ng/ml (serum NSE level) 1 week after TBI, and the patient eventually died.
Discussion
In this pilot study, serial blood measurements of S100B and NSE were investigated to evaluate the severity of initial brain damage after TBI and to predict the clinical outcome. The poor consciousness in children after trauma had a significant effect on the serum S100B and NSE levels. After 1 week of brain trauma, a statistically significant decrease was observed in the serum S100B and NSE levels, but they increased in one patient, with a fatal outcome.
S100B and NSE as biomarkers
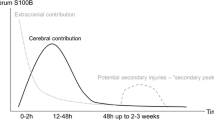
The first human TBI study of the value of S100B as a serum biomarker of brain injury assessment was published by Ingebrigtsen et al. in 1995 [5]. Later, it became known as being effective for assessing various brain injury lesions. The release of S100B from the central nervous system (CNS) suggests that low S100B serum levels are consistent with blood-brain barrier (BBB) opening without damage of neuronal tissue, whereas a larger increase implies release from damaged glia [6]. As in the above study, our results showed lower S100B serum level in the favorable group consisting of GCS 14 and 15 and higher S100B level in the unfavorable group consisting of GCS 4 and 9. The physiological functions of S100B have been shown to be concentration dependent, where lower concentrations are beneficial and higher concentrations correlate to harmful effects [7]. NSE is a biomarker of neuronal damage. Early NSE levels have been correlated with outcome in children, particularly those younger than 4 years of age [1]. In a comparative study of S100B and NSE, S100B was a better outcome predictor overall, with NSE only predicting mortality [8]. NSE has a longer half-life than S100B, while S100B is related to extracranial injury. Therefore, NSE may be useful in TBI patients with multiple trauma. The limitation of NSE is the occurrence of false-positive results in the setting of hemolysis [9]. TBI causes leaking of S100B and NSE from damaged brain cells into the blood or cerebrospinal fluid (CSF). The concentrations of biomarkers in the CSF might be more accurate for outcome prediction in TBI. However, biomarkers measurement in the CSF could not demonstrate statistical significance, probably due to its heightened sensitivity to the smallest of influences [10].
In our study, serially measured serum S100B and NSE levels at within 6 h and 1 week after trauma showed a significant positive correlation (r = 0.898). A positive correlation between S100B and NSE might indicate injury of both glial and neuronal cells [11]. Kruijk et al. [12] found a strong correlation between S100B and NSE (r = 0.5) in patients of whom the S100B and NSE serum levels were increased. In patients with mild TBI, a weaker correlation (r = 0.258) between S100B and NSE has been reported [13]. S100B and NSE are released not only from the CNS, but also from extracranial system. An elevated S100B level was reported in patients with extracranial injury without TBI [14]. However, another report has also shown that the S100B level does not affect on patients with extracranial injury [12]. Our results showed that the presence of concomitant extracranial injury had no significant effect on S100B and NSE. Serum S100B released from an extracranial origin appears to have a faster clearance than S100 released from the CNS [14]. When using biomarkers to evaluate TBI patients, the extracranial factors should be considered. The extracranial factors could be excluded using repeated measurement after 12 h, considering their half-life. S100B and NSE have a significant negative correlation with age. Gazzolo et al. [15] found a rapid decrease from 0 to 7 years followed by an increase from 7 to 13 years and then by a second decrease from 14 to 15 years of age. We did not find any correlation between S100B and NSE levels and age, probably because of the small number of patients. Serum S100B presents higher level in the focal, more severe brain injuries, such as cerebral contusion and subdural hematomas, compared to more diffuse injuries [10]. Also, S100B is a good marker of BBB permeability. Understanding different types of TBI with proper brain imaging is necessary for clinical use of S100B. Recently, Thompson et al. [16] suggested that functional MRI connectivity correlate with serum levels of the S100B protein in the acute phase of TBI.
S100B and NSE in mild and moderate to severe TBI patients
S100B and NSE have been most commonly studied for evaluation of brain damage. These protein biomarkers can be used as a diagnostic tool for prediction of clinical outcome and monitoring secondary injury development. A significant negative correlation has been shown between serum S100B and NSE levels and the severity of injury assessed using GCS in pediatric patients with mild to moderate TBI [1, 17, 18]. A wide range of brain damage may be helpful from patients with minor injuries to those who are unconscious. The initial measurement of serum biomarkers after trauma is considered the most important for outcome prediction [19]. Our results showed that in the favorable group, the serum S100B and NSE levels measured at admission were low and we predicted a mild degree of brain damage. One week later, the measured serum S100B and NSE levels were lower than those at admission, and the GOS scores at 6 months were reported as good recovery. Biomarkers are also helpful in unconscious patients in need of neuro-intensive care. For severe TBI patients at the time of acute care, secondary brain damage or ongoing brain injury should be monitored and routine radiological examination including brain CT may be limited. At this time, the serum biomarker of brain damage can help as surrogate markers in the treatment and monitoring of brain injury. Four patients in the unfavorable group of five patients with stuporous or semi-comatose mentality showed a decrease in serum S100B and NSE levels 1 week after admission and a good GOS score of 4 (moderate disability but independent) or 5 (good recovery). However, one patient with increased S100B and NSE serum levels 1 week later had a fatal result with a GOS score of 1 (death). The half-life of S100B has been shown to be in the range of 1 to 2 h in patients with TBI [20]. Because of the short half-life of serum S100, a steady increase in S100B indicates sustained inflow, i.e., ongoing brain injury. Our results showed that serum S100B level 1 week after TBI in the unfavorable group was higher than that in the favorable group, with significant differences. Serum NSE at admission and 1 week after trauma or S100 at admission levels in the unfavorable group were higher than those in the favorable group, without statistical differences. The serial measurement of serum biomarker should be considered to increase the accuracy of the test.
S100B and NSE in pediatric patients
Increased serum biomarker levels are correlated with secondary brain damage. To detect ongoing brain injury, brain CT or ICP monitoring are used in neuro-intensive units. Korfias et al. [21] reported neurological deteriorations not detected using ICP monitoring and a secondary increase of serum biomarkers. Serum biomarkers can be used instead of ICP monitoring in pediatric TBI patients who are reluctant to monitor with ICP probe due to invasiveness or economic reasons. In the 2013 Scandinavian CT guidelines for mild and moderate TBI patients, if the concentration is less than 0.10 μg/l within 6 h after trauma and the patient is suffering from a mild TBI without extracranial trauma, avoiding performing a head CT scan could be considered [22]. In theory, this can reduce the number of unnecessary CT scans by about one third. Serum S100B measurement may be helpful for mild TBI screening in the emergency room when patients worry about radiation exposure and CT scan cost, or if intoxicated patients need sedation and intubation. Pediatric TBI patients may benefit most when serum biomarkers are used instead of unnecessary CT scans, because radiation from the CT scans is particularly harmful for children. Serum S100B and NSE are used as a surrogate marker of TBI in children and adults. Berger et al. [23] reported that in severe pediatric TBI patients, increasing serum levels show a higher risk of unfavorable outcome compared to patients with subsequent serum concentrations in steady decline. Other authors suggest that these biomarkers can provide valuable information about brain damage in preterm newborns and hypoxic-ischemic injuries [24, 25].
The main limitations of our study are the small data set and insufficient serial blood measurements. The normal range of serum S100B and NSE levels in the pediatric group was not presented in the ELISA test kits used in this study. Pediatric patients who arrived more than 6 h after brain trauma were excluded from this study. The time range from trauma to hospital arrival was thus narrow. Analysis of our results may have been limited by the small amount of data. Two blood measurements immediately after trauma and at 1 week after trauma may not have been sufficient to observe a change in serum biomarker levels after TBI. Sufficient serial serum biomarkers data in pediatric patients with TBI are needed to establish them as good indicators for brain damage.
Conclusions
Elevated serum S100B and NSE levels in pediatric patients within 6 h after TBI decreased at 1 week after trauma. These indicators were higher in pediatric patients with poor consciousness at the beginning of the brain trauma. This was a preliminary study on the potential association between serum S100B and NSE levels and pediatric TBI. In particular, serum S100B 1 week after trauma seems to be closely related to the severity of brain damage. Our results have implications for further research on determining whether serum S100B and NSE levels in pediatric TBI patients could be useful to predict the clinical outcome and know the severity of brain damage by serial measurements.
References
Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM (2005) Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J Neurosurg 103:61–68
Ingebrigtsen T, Romner B (2002) Biochemical serum markers of traumatic brain injury. J Trauma 52:798–808
Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, Zimmerman C, van Geel W, de Reus H, Biert J, Verbeek MM (2004) Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 62:1303–1310
Beers SR, Berger RP, Adelson PD (2007) Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma 24:97–105
Ingebrigtsen T, Romner B, Kongstad P, Langbakk B (1995) Increased serum concentrations of protein S-100 after minor head injury: a biochemical serum marker with prognostic value? J Neurol Neurosurg Psychiatry 59:103–104
Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, Kanner A, Ayumar B, Albensi B, Cavaglia M, Janigro D (2003) Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci 21:109–121
Rothermundt M, Peters M, Prehn JH, Arolt V (2003) S100B in brain damage and neurodegeneration. Microsc Res Tech 60:614–632
Thelin EP, Jeppsson E, Frostell A, Svensson M, Mondello S, Bellander BM, Nelson DW (2016) Utility of neuron-specific enolase in traumatic brain injury; relations to S100B levels, outcome, and extracranial injury severity. Crit Care 20:285
Ramont L, Thoannes H, Volondat A, Chastang F, Millet MC, Maquart FX (2005) Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clin Chem Lab Med 43:1215–1217
Kellermann I, Kleindienst A, Hore N, Buchfelder M, Brandner S (2016) Early CSF and serum S100B concentrations for outcome prediction in traumatic brain injury and subarachnoid hemorrhage. Clin Neurol Neurosurg 145:79–83
Herrmann M, Jost S, Kutz S, Ebert AD, Kratz T, Wunderlich MT, Synowitz H (2000) Temporal profile of release of neurobiochemical markers of brain damage after traumatic brain injury is associated with intracranial pathology as demonstrated in cranial computerized tomography. J Neurotrauma 17:113–122
de Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Twijnstra A (2001) S-100B and neuron-specific enolase in serum of mild traumatic brain injury patients. A comparison with health controls. Acta Neurol Scand 103:175–179
Stålnacke BM, Björnstig U, Karlsson K, Sojka P (2005) One-year follow-up of mild traumatic brain injury: post-concussion symptoms, disabilities and life satisfaction in relation to serum levels of S-100B and neurone-specific enolase in acute phase. J Rehabil Med 37:300–305
Savola O, Pyhtinen J, Leino TK, Siitonen S, Niemelä O, Hillbom M (2004) Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J Trauma 56:1229–1234
Gazzolo D, Michetti F, Bruschettini M, Marchese N, Lituania M, Mangraviti S, Pedrazzi E, Bruschettini P (2003) Pediatric concentrations of S100B protein in blood: age- and sex-related changes. Clin Chem 49:967–970
Thompson WH, Thelin EP, Lilja A, Bellander BM, Fransson P (2016) Functional resting-state fMRI connectivity correlates with serum levels of the S100B protein in the acute phase of traumatic brain injury. Neuroimage Clin 12:1004–1012
Bandyopadhyay S, Hennes H, Gorelick MH, Wells RG, Walsh-Kelly CM (2005) Serum neuron-specific enolase as a predictor of short-term outcome in children with closed traumatic brain injury. Acad Emerg Med 12:732–738
Cotena S, Piazza O, Storti M (2006) The S100B protein and traumatic brain injury. J Neurosurg 104:435–436
Jackson RG, Samra GS, Radcliffe J, Clark GH, Price CP (2000) The early fall in levels of S-100 beta in traumatic brain injury. Clin Chem Lab Med 38:1165–1167
Ingebrigtsen T, Romner B (2003) Biochemical serum markers for brain damage: a short review with emphasis on clinical utility in mild head injury. Restor Neurol Neurosci 21:171–176
Korfias S, Stranjalis G, Boviatsis E, Psachoulia C, Jullien G, Gregson B, Mendelow AD, Sakas DE (2007) Serum S-100B protein monitoring in patients with severe traumatic brain injury. Intensive Care Med 33:255–260
Undén L, Calcagnile O, Undén J, Reinstrup P, Bazarian J (2015) Validation of the Scandinavian guidelines for initial management of minimal, mild and moderate traumatic brain injury in adults. BMC Med 13:292
Berger RP, Bazaco MC, Wagner AK, Kochanek PM, Fabio A (2010) Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev Neurosci 32:396–405
Graham EM, Everett AD, Delpech JC, Northington FJ (2018) Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr Opin Pediatr 30:199–203
Serpero LD, Pluchinotta F, Gazzolo D (2015) The clinical and diagnostic utility of S100B in preterm newborns. Clin Chim Acta 444:193–198
Funding
This work was supported by Biomedical Research Institute grant (No.2016-General-08), Kyungpook National University Hospital (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, DW., Park, SH. & Hwang, SK. Serial measurement of S100B and NSE in pediatric traumatic brain injury. Childs Nerv Syst 35, 343–348 (2019). https://doi.org/10.1007/s00381-018-3955-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-018-3955-y