Abstract
Purpose
Ventriculo-peritoneal (VP) shunts are effective for treatment of hydrocephalus in all age groups; however, they are associated with complications, a common one being ventricular catheter (VC) obstruction. VC position is likely to influence VC survival; however, most VCs are positioned freehand without guidance. This paper describes the accuracy of ultrasound guidance for VC placement and the impact of tip location on VC occlusion rate.
Methods
This is a retrospective cohort study of hydrocephalic children with first-time VP shunt and ultrasound-guided VC placement. Data recorded were age, sex, cause of hydrocephalus, side (left or right) and location (frontal or occipital) of VC, and exact postoperative position within the ventricle on first postoperative imaging: middle of ventricle (optimal position), near or touching the medial or lateral ventricle wall, within the third ventricle, and at the contralateral side.
Results
Of the 128 screened patients, 85 had a first postoperative imaging that clearly defined the VC position and were included. The follow-up was at least 12 months. Seventy-three percent of VCs were placed on the right and 71% via a frontal burhole. Eighty-three of 85 VC tips (95%) were in the intended ventricle, 61% at optimal position. Nine of 85 VCs (10%) obstructed within the first 12 months. Seven of nine (78%) obstructed VCs were located in a nonoptimal position (p = 0.016). Two of nine (22%) obstructed VCs entered through a frontal and seven of nine (78%) through an occipital burrhole (p = 0.016).
Conclusion
Ultrasound-guided VC placement is as precise as frameless navigated placement. The optimal VC position was associated to a significant lower VC obstruction rate. The frontal position was superior to the occipital. Intraoperative US guidance is fast with almost no extra time and no extra cost. US-guided VC placement should become standard of care in VP shunt surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is the most common surgically treatable neurological disorder in children from infancy through adolescence [1]. Treatment can either be endoscopic third ventriculoscopy (ETV) if certain requirements are fulfilled or the implantation of a ventricular shunt (VS) that drains excessive cerebral fluid into the heart (ventricular atrial shunt) or the abdomen (ventricular peritoneal shunt). Ventricular shunting is prone to several types of shunt failure. Shunt obstruction—often manifested as ventricular catheter obstruction—is one of the primary causes for shunt revisions resulting in an average of 2.7 revisions per patient with congenital hydrocephalus at the age of 17 [2]. Ventricular catheter (VC) obstruction can be related to its position in the ventricle. If the tip of the VC is located away from the ventricular wall and the choroid plexus, it is suggested that shunt survival would improve [3] as it is believed that obstruction by the choroid plexus is the most common reason for failure of the proximal catheter [4]. Most VC placements are done freehand using anatomical landmarks. Wilson et al. showed that the use of neuronavigation and ultrasound increased the accuracy of the catheter location in the ventricular system (89% were accurately placed) and reduced the proximal shunt failure rate in an adult cohort. The study identified the freehand technique by using only anatomical landmarks as the only risk factor for inaccurate placement [5]. Whitehead et al. [6] used ultrasound guidance in catheter placement in older pediatric patients with closed fontanelle to intraoperatively ensure a location away from the choroid plexus. There was, however, no analysis of the long-time shunt survival in these patients.
An analysis of three large pediatric studies was unable to identify an optimal VC position within the ventricle that influenced the shunt survival. Only the location of VC entry, whether anterior or posterior, was important [7].
This study evaluates the benefit of intraoperative ultrasound guidance for first-time VC placement in children by postoperative verification of the VC position and investigates proximal shunt survival with respect to the VC position within the ventricle.
Methods
Study design
This is a retrospective cohort study of children with hydrocephalus treated at the University Hospital of Tuebingen in Germany between April 2009 and October 2015. Patients were identified from the pediatric neurosurgical database. Inclusion criteria were first-time VP shunt insertion and VC positioning performed with intraoperative ultrasound guidance. All shunts were equipped with a gravity-assisted valve system (proGAV 0-20/25, Miethke, Potsdam, Germany) to minimize the risk of overdrainage.
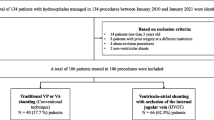
One hundred twenty-eight first-time shunt insertions were identified. Of those, 85 patients were included in the final analysis in which early postoperative intracranial imaging (ultrasonography, MRI) clearly identified the location of the VC. The baseline demographics included age, sex, underlying cause of hydrocephalus, and side (left or right) and location (frontal or occipital) of VC entry. Table 1 provides the baseline characteristic of the population of the study.
Ventricular catheter placement technique
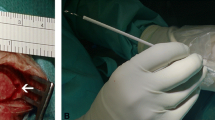
The placement of the ventricular catheter was performed with an Aloka® ultrasound machine model Prosound SSD-3500SX and a burhole ultrasound probe (Fig. 1). After the creation of the burhole at the desired frontal or occipital location (entry point determined according to MRI criteria), the catheter length was determined by ultrasound measurement, aiming at a maximum length without touching the ventricular floor (frontal location) or opposing wall (occipital location). The catheter was cut to the desired length and connected to a burhole reservoir. The insertion trajectory was determined by bringing the coronal (frontal burhole) or axial (occipital) ultrasound scan into a position, where the target region was on the vertical 12 to 6 o’clock line corresponding to the trajectory (Fig. 2a). Catheter length and trajectory were chosen to place the catheter tip to the target area, which was in the lower middle of the desired frontal horn, which was considered to be the best possible position (see “Imaging and scoring system”). Then, a Cushing cannula was placed in the guiding indentation of the ultrasound probe, taking up the ideal trajectory orientation of the ultrasound. After removing the ultrasound probe from the burhole, the cannula orientation was maintained and the cannula was inserted and advanced to the ventricle in the predetermined orientation. As soon as CSF was dripping, the cannula was withdrawn and the ventricular catheter was fixed to a burhole reservoir inserted along the created path.
a Insertion of the ventricular catheter with closed fontanelle at a frontal position: The burhole ultrasound probe (US) is placed over the burhole, and the trajectory (dashed line) is adjusted to aim to the middle of the frontal horn just anterior to the foramen of Monroe. The length of the VC is measured and cut so its tip will be positioned in the lower third of the frontal horn without touching walls or reaching into the foramen of Monroe after implantation. Then, the “Cushing” cannula (CN) is pushed forward exactly in this trajectory (asterisk) to puncture the ventricle after removing of the US. When CSF is dripping, the cannula is removed and the VC inserted along the preformed path. b Insertion of the ventricular catheter with open fontanelle at a frontal position: the burhole ultrasound probe is placed on to the fontanelle so that both ventricles are visualized. The length of the VC is determined by ultrasound measurement and cut. The “Cushing” cannula is then inserted under constant visualization aiming towards the middle of the lower third of the frontal horn just anterior to the foramen of Monroe. After removal of the cannula, the VC can be inserted and position again be controlled by ultrasound
In children with a still open fontanelle, the technique was modified in the way that the ultrasound was placed over the fontanelle and the Cushing cannula advanced from the lateral burhole under direct vision into the desired position in the middle of the ventricle (Fig. 2b).
Imaging and scoring system
The first postoperative cranial imaging where the ventricular catheter tip could be clearly identified was evaluated. Preferably, this was performed within the next 48 h postoperatively using ultrasound in children with an open fontanel. In children with closed fontanelle, a follow-up MRI which was usually performed within 3–6 months postoperatively was used as first imaging.
The “intended ventricle” is the ventricle that the surgeon anticipated to hit. To grade the position of the ventricular catheter, the following scoring system was applied with regard to the location of the tip of the ventricular catheter
-
Near (surrounded by CSF) or touching the medial ventricle wall
-
Near (surrounded by CSF) or touching the lateral ventricle wall
-
Middle of the ventricle (optimal position)
-
Within the third ventricle
-
At the contralateral side
Figure 3 shows the grading system with regard to the location of the tip of the ventricular catheter in the postoperative cranial imaging.
Scoring system according to the location of the tip of the ventricular catheter in the postoperative cranial imaging. Examples of MRI imaging: a tip touching the medial wall of the ventricle, b tip touching the lateral wall, c optimal position where the tip is located in the middle of the punctured ventricle surrounded by cerebral spine fluid and neither near nor touching walls, d tip located in the third ventricle, and e tip of the ventricle catheter lies in the contralateral side of the punctured ventricle
Follow-up
The clinical routine follow-up was done according to the usual protocol, and follow-up information was taken from the medical records. Failures of the shunt system due to a ventricular catheter obstruction were noted. More distal system failures due to the valve or abdominal catheter malfunction were not taken into analysis since they were considered to be independent of ventricular catheter position. We divided ventricular catheter occlusions in early within the first 3 months and late occlusion between 3 and 12 months postoperatively.
The chi-square test was used to test for statistical significance in comparisons of all the categorical data as well as the Fisher exact test when applicable. Statistical significance was considered at p < 0.05.
Results
The basic data of the 85 analyzed pediatric patients (mean age 35 months, 2.9 years, range newborn (0 days) to 148 months, 12 years) is shown in Table 1. It includes age, gender, diagnosis, side of the ventricular positioning (left vs. right), and location of entry point (fontal vs. occipital). They had a follow-up of at least 12 months. The period of time between operation and first available image differed widely from within 48 h after the operation (in all children with open fontanelle) to MRIs conducted as late as 1 year postoperatively in older children with uneventful postoperative course.
Sixty-two ventricular catheters (73%) were implanted through a right-sided burhole versus 23 (27%) through a left burhole. Sixty patients (71%) received a frontal and 25 (29%) an occipital VC (Table 1). In all 85 patients, the intended ventricle was hit in the first attempt with the Cushing cannula and all VCs could be inserted easily through the cannula channel at first attempt.
In the first postoperative cranial imaging, 52 catheters (61%) were positioned in the middle of the intended ventricle which is considered the optimal position. Twenty-three (27%) were close or touching the medial wall, and eight (9%) VC tips were close or touching the lateral wall of the ventricle. Two catheters were too long: in one, the tip of the catheter was located within the third ventricle, in the other, at the opposite side (see Fig. 3). Thus, in 83 of 85 cases (97.6%), the VC tip was in the intended ventricle (Table 2).
The follow-up of all patients was at least 12 months. During these first 12 months, nine patients (10.6%) had a ventricular catheter occlusion. Five (55%) had an early and four (45%) a late occlusion (Table 3). In two of the nine obstructed VCs (22%), the initial tip location was in the middle (optimal position), five of nine (55%) were located close or touching the medial wall, and two of nine (22%) close or touching the lateral wall. Two of nine VCs (22%) were placed through a frontal and seven of nine (78%) through an occipital burhole (Table 3).
Occlusion rates were the same for right-sided VCs (7/62, 11%) and left-sided VCs (2/23, 9%) (p 0.54).
When comparing frontal vs. occipital insertion, there was a statistically significant lower occlusion rate for frontal VCs (2/60, 3%) vs. occipital VCs (7/25, 28%), p < 0.002.
Furthermore, an optimal VC position was associated to a significant lower occlusion rate (2/52, 4%) compared to all other nonoptimal positions (7/33, 21%), p < 0.016.
No statistical significance was seen for early vs. late occlusion with regard to frontal or occipital entry and optimal versus nonoptimal positions, respectively.
Discussion
Shunting operations are one of the most frequent neurosurgical procedures performed in children. Despite the fact that all freehand techniques using anatomical landmarks have a known 12.5 to 40% inaccuracy of catheter placement [8,9,10], this is still the most common practice. Reasons for not using guidance systems might be the time-consuming preparation and necessity of suitable imaging datasets for frameless neuro-navigation procedures and the problem of availability of these techniques for a shunt procedure [11]. Another aspect is thought to be the lack of awareness that the catheter misplacement rate is high and tip location relevant for shunt survival [12]. Several studies have already shown that the use of transcranial ultrasound through the fontanelle of younger children is helpful in VC placement [7, 13,14,15]. A more recent study with, however, only ten older pediatric patients with an already closed fontanelle used real-time ultrasound guidance and in all cases, the VC was placed in the intended ventricle confirmed by a CT scan [6].
Our study is the largest published series of ultrasound-guided VC placements in a purely pediatric population, where ultrasound was used independent of the patient’s age or existence of an open fontanelle, since our employed techniques works with a bony opening of 6–8-mm diameter which is not larger than the opening usually created for shunt placement.
Our technique for closed fontanelle uses ultrasound “only” to precisely determine the trajectory and bringing the Cushing cannula into exact this orientation. The actual puncture was then carried out without visual ultrasound control of the cannula as opposed to navigating the cannula/catheter under continuous ultrasound control into the ventricle, which is possible only with an open fontanelle. Nevertheless, we obtained a 100% rate of catheter placement in the intended ventricle. Because two VCs were too long and thus ended in the third and the opposite ventricles, there was only a 95% rate of placement of the VC tip in the desired ventricle, with 61% in optimal position (without being close to or touching walls or choroid plexus).
The ultrasound-guided VC placement procedure as described has a steep learning curve and is a “must-do” part of all shunt implantations in our department. It is trained to all residents from their first shunt surgery they assist in adults as in children and thus is routine to all involved.
In all cases without open fontanelle, the next intracranial imaging was routinely carried out within 3–6 months postoperatively, in rare cases up to 1 year later. Thus, in many of those children, the ventricular size had come down in the meantime and initially optimal placed VCs might now have been in a more lateral or medial position than immediately postoperative. It can thus be assumed that the rate of initially optimally placed VCs was rather higher than the determined 61%.
It could previously be shown that the use of frameless navigation- and ultrasound-improved the accuracy of VC placement in adults and children with and without open fontanelles: Hayhurts et al. had a success rate of 100% of VC positioned in the intended ventricle using electromagnetic navigation, with 74% free-floating and 24% close or touching the ventricle wall [16] in children and adults. In contrary to our study, where imaging was done according to routine protocol in those cases with closed fontanelle later, the authors obtained a control imaging immediately after surgery in all cases. The pediatric cohort was not analyzed separately; therefore, it is unclear if electromagnetic navigation in their hands was actually superior to the results of this study. Wilson et al. compared the accuracy of ventricular catheter placement in adults using ultrasound and navigation and had an 89% and respectively 88% rate of the intended ventricle position [5]. Our study in an entirely pediatric population compares to these results with 95% of the VC tip placed into the intended ventricle.
The rate of VC lying postoperatively within the intended ventricle is one measurement of success of placement. Another one and more precise is the evaluation of the exact location of the VC tip within the intended ventricle, as it is suggested that the position of VC tip away from the ventricular wall and the choroid plexus would improve shunt survival [3]. In our study, we went even further and evaluated how the position of the CV tip correlates with obstruction up to 12 months postoperatively. Within this follow-up, nine patients (10%) experienced VC occlusion, five of them within 3 months rather early. Evaluation of the first postoperative imaging showed that the VC occlusion rate was low (4%) if the VC tip initially had an optimal position. In a nonideal tip position, the occlusion rate was more than five times higher (21%). Similarly, the occlusion rate was almost threefold higher in case an occipital catheter entry was chosen (78%) as opposed to frontal entry (22%) Thus, occipital burhole placement and nonideal catheter tip location seem to be risk factors for catheter occlusion within 12 months of surgery.
A similar analysis of catheter tip position to VP shunt occlusion rate in a pediatric group (age < 18 years) was performed by Whitehead et al. in 2017. The authors analyzed three pediatric studies with and without ultrasound-guided VC placement, however, failed to identify an optimal position of the VC with regard to overall shunt failure due to obstruction, over drainage, loculations, or infection. The authors, however, as well suggested that the occipital entry position might be a risk factor for shunt failure [7].
Our study seems to indicate that an occipital entry point carries a much higher risk of VC obstruction than a frontal burhole. Although this was statistically significant in our cohort, the analysis is limited by the fact that we did not use a randomized protocol regarding a frontal versus occipital entry point. The choice of the entry point was surgeon dependent (with an initial preference of the senior author for occipital position) and was chosen more often in the earlier years of the study. The reason for performing less occipital VCs over time was the subjectively “sensed” higher numbers of VC obstructions in these patients, although a true retrospective analysis was not performed prior to this study.
As discussed above is the considerable variability regarding the time point for the postoperative imaging of the catheter position a limitation of our study. After closure of fontanelle, all children received a first routine MRI within 6–12 months after surgery or after closure of the fontanelle. All children included in this study had at least one postoperative MRI. However, those with open fontanelle at surgery were imaged before discharge by trans-fontanelle ultrasound. Unfortunately, sonographers not always clearly depicted the VC tip location in their documented scans. Therefore, 43 infants had to be excluded from the analysis, since the initial postoperative ultrasound images did not allow for a precise description of the VC tip location. Therefore, as already mentioned, the ideal initial catheter placement rate might have been higher than documented if all children would have received a MRI before discharge. This could not be realized for logistic reasons and the necessary sedation/intubation in small children would not have been justifiable just for the curiosity of knowing the VC tip location in children with a working shunt and being well. CT scans for this purpose are considered unethical because of the associated risks of radiation-induced malignancies in very small children and have been abandoned in our service for nonemergency imaging for years.
Concerning intraoperative practicability and time requirements the use of ultrasound has many advantages over frameless navigated procedures. First of all, there is no necessity for suitable thin-sliced and recent MRI or CT datasets as the necessary basis for infrared or electromagnetic navigation systems. This saves cost and sedation for infants and smaller children on the imaging side as well as time before surgery for planning and setup of the navigation system in the OR.
Second, ultrasound has virtually no extra setup time except for the sterile draping of the probe. There is one quick (< 20 s) scan for anatomical orientation and determination of catheter length, and a second scan for determination of the optimal trajectory. The second scan takes, together with positioning of the Cushing cannula within the guiding indentation of the ultrasound probe, another 30 s. Thus, the workflow of shunt implantation is virtually unchanged as compared to a freehand ventricular puncture. The use of ultrasound does not prolong or complicate the procedure and thus is, because operation time is not prolonged, no risk factor regarding shunt infection. The same applies to the use of another foreign body, the ultrasound probe, at shunt surgery. Certainly, sterile draping of the burhole probe has to be performed correctly. We did not administer a prolonged course of antibiotics, all patients did receive a routine single shot of cefuroxime in the dose of 100 mg/kg. In this cohort, we did not see an increase infections rate, which was 1/85 children. This is consistent with other studies [6].
Conclusion
Ultrasound-guided VC placement is quick and safe and has no extra costs if an ultrasound machine and a probe is already present, apart from a sterile ultrasound probe drape. Compared to a frameless infrared or electromagnetic navigation system, an ultrasound system with a burhole probe is certainly not more expensive.
We could furthermore show that ultrasound in trained hands is as precise as navigated procedures and the learning curve for ultrasound application is steep in our experience. The use of ultrasound seems to prolong VC survival as optimized catheter placement was associated to a much lower rate of catheter obstruction. At least in our hands, the frontal entry point was superior regarding VC obstruction rates, which might mostly be due to the easier targeting of the frontal horn anterior to the foramen of Monroe as compared to the occipital entry.
Therefore, we strongly recommend ultrasound use as a routine tool for shunt catheter placements and guided implantation of VCs (by whatever means) as standard of care. Since shunt revisions are the major “curse” of hydrocephalus treatment, all avoidable cofactors leading to shunt revision have to be eliminated. Nonoptimal VC position is certainly one of them.
References
Flannery AM et al (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. J Neurosurg Pediatr (Suppl) 14:1–2
Lumenta CB, Skotarczak U (1995) Long-term follow up in 233 patients with congenital hydrocephalus. Childs Nerv Syste 11:173–175
Kemp J, Flannery AM, Tamber MS, Duhaime AC (2014) Systematic literature review and evidence- based guidelines. Part 9: effect of ventricular entry point and position. J Neurosurg (Suppl) 14:72–76
Korinek AM, Laurence F-O, Boch AL, Puybasset L (2011) Morbidity of ventricular cerebrospinal fluid shunt surgery in adults: an 8-year study. Neurosurgery 68:985–995
Wilson TJ, Stetler WR, Al-Holou WN, Sullivan SE (2013) Comparison of the accuracy of ventricular catheter placement using freehand placement, ultrasonic guidance, and stereotactic neuronavigation. J Neurosurg 119:66–70
Whitehead WE, Jea A, Vachhrajani S, Kulkarni AV, Drake JM (2007) Accurate placement of cerebrospinal fluid shunt ventricular catheters with real-time ultrasound guidance in older children without patent fontanelles. Technical note. J Neurosurg 107(5 Suppl):406–410
Whitehead WE et al (2017) Ventricular catheter entry site and not catheter tip location predicts shunt survival: a secondary analysis of 3 large pediatric hydrocephalus studies(2):157–167
Hayhurts et al (2010) Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. J Neurosurg 113(6):1273–1278
Toma AK, Camp S, Watkins LD, Grieve JP, Kitchen N (2009) External ventricular drain insertion accuracy: is there a need for change in practice? Neurosurgery 65:985–986
Hsieh CT et al (2011) The misplacement of external ventricular drain by freehand method in emergent neurosurgery. Acta Neurol Belg 111:22–28
Huyette DR, Turnbow BJ (2008) Accuracy of the freehand pass technique for ventriculostomy catheter placement: retrospective assessment using computed tomography scans. J Neurosurg 108:88–91
Sampath R, Wadhwa R, Tawfik T, Nanda A, Guthikonda B (2012) Stereotactic placement of ventricular catheters: does it affect proximal malfunction rates? Stereotact Funct Neurosurg 90–97
Babcock DS, Barr LL, Crone KR (1992) Intraoperative uses of ultrasound in the pediatric patient. Pediatr Neurosurg 18:84–91
Kellnar S, Ring-Mrozik E, Deindl C (1989) Intraoperative sonographic diagnosis of the ventricular position of shunt systems in infants with hydrocephalus. Z Kinderch 44:131–134
Ruge JR, Dauser RC, Storrs BC (1991) Posterior fossa cysts: supratentorial shunt placemen with ultrasound guidance. Childs Nerv Syst 7:165–168
Shkolnik A, McLone DG (1981) Intraoperative real-time ultrasonic guidance of ventricular shunt placement in infants. Radiology 141:515–517
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I, Marcel Kullmann, hereby confirm that there is no conflict of interest with regards to the data presented in the manuscript (“Ultrasound-Guided Placement of Ventricular Catheters in First Time Pediatric VP-Shunt Surgery”). There are no financial and personal relationships with other people or organizations that influence (bias) my work inappropriately.
Rights and permissions
About this article
Cite this article
Kullmann, M., Khachatryan, M. & Schuhmann, M.U. Ultrasound-guided placement of ventricular catheters in first-time pediatric VP shunt surgery. Childs Nerv Syst 34, 465–471 (2018). https://doi.org/10.1007/s00381-017-3660-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3660-2