Abstract
Background and purpose
To test the hypothesis that the prevalence of cerebral cavernous malformation (CCM) associated with developmental venous anomalies (DVAs) increases with age, we studied the age-related prevalence of DVA-associated CCM among patients with DVAs.
Materials and methods
Patients with DVAs on contrast-enhanced MRI exams performed over a 2-year period were included in this study. A single neuroradiologist reviewed all imaging exams for the presence of CCMs. Baseline demographic data collected included age, gender, presence of CNS neoplasm, history of cranial radiation, and history of seizure. Patients were divided into age groups based on decade of life. Cochran-Armitage trend tests were performed to determine if increasing age was associated with CCM prevalence.
Results
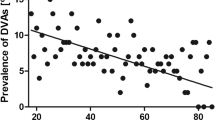
A total of 1689 patients with DVAs identified on contrast-enhanced MRI were included. Of these patients, 116 (6.9%) had a cavernous malformation associated with the DVA. There was a significant positive association between age and the prevalence of DVA-associated CCM (P = 0.002). The prevalence of DVA-associated CCM was 0.8% for the 0–10 age group, 1.6% for the 11–20 age group, 7.5% for the 21–30 age group, 9.5% for the 31–40 age group, 6.1% for the 41–50 age group, 6.3% for the 51–60 age group, 7.4% for the 61–70 age group, and 11.6% for the >70 age group (P < .0001).
Conclusions
Our study demonstrated an age-related increase in prevalence of DVA-associated cavernous malformations among patients with DVAs. These findings suggest that DVA-associated cavernous malformations are acquired lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pathogenesis and timing of cerebral cavernous malformation (CCM) development among patients with developmental venous anomalies (DVAs) is unclear. Interestingly, up to 25% of CCMs are associated with DVAs [1, 2] on standard sequences, and more recently, smaller studies have found that up to 100% of CCMs are associated with a DVA on 7T imaging [3]. A number of studies and case reports have demonstrated de novo formation of CCM in patients with DVA, thus suggesting that DVA-associated CCMs are not congenital lesions, but rather develop due to changes in DVA venous architecture or physiology [2, 4,5,6]. However, it remains unclear whether de novo CCM formation in the setting of a DVA is the exception rather than the rule.
While a review of the literature suggests that DVA-associated CCMs are less common in children than in adults [7,8,9,10], to date, there have been no large studies examining the age-related prevalence of DVA-associated CCMs. If the prevalence of CCMs increases with age among patients with DVAs, it would be reasonable to assume that these lesions are not congenital and may in fact form due to changes in DVA architecture and physiology. In this study, we examined the age-related prevalence of DVA-associated CCMs in order to test the hypothesis that the prevalence of DVA-associated CCMs increases with age. Findings from this study could provide insight into the pathogenesis of DVA-associated CCMs.
Materials and methods
Patient selection
Following IRB approval, we reviewed reports from a consecutive series of patients diagnosed with brain developmental venous anomalies on contrast-enhanced head MRIs performed over a 2-year (2014–2015) period at our institution. All patients were scanned on 1.5T or 3.0T scanners, and all patients had some form of post-contrast T1-weighted scan of the entire brain, a pre-contrast T1-weighted scan, a T2-weighted scan, and T2*-weighted imaging (i.e., gradient recall echo or susceptibility-weighted imaging).
Data collection
One neuroradiologist examined all MRI imaging on patients with DVA to assess for concomitant CCM. CCMs had to meet the imaging criteria put forth by Zambramski et al. [11] and were categorized as follows: Type 1: subacute hemorrhage which is T1 hyperintense and T2 hyperintense or hypointense, Type 2: classic “popcorn” lesion with mixed T1 and T2 signal centrally and low signal rim with blooming on T2* sequences, Type 3: chronic hemorrhage which is T1 hypointense or isointense centrally, T2 hypointense centrally and has a low signal rim with blooming on T2* sequences, and Type 4: difficult to identify on T1 or T2, but apparent on T2* as a black dot with blooming. In order for the cavernous malformations to be considered to be associated with the DVA, they had to be centered around the venous radicals or main draining vein of the DVA. Type 4 lesions seen in close proximity of a DVA without any other pattern of microbleed suggestive of another etiology (i.e., amyloid angiopathy, vasculitis) were included. Any potential CCMs located distant from the DVA or outside the DVA draining territory were not included in this prevalence analysis.
Outcomes
The primary outcome of this study was prevalence of DVA-associated CCM by age group. Patients were divided into age groups by decade of life. Data on gender, DVA location, seizure history, central nervous system neoplasm history, and history of prior cranial radiation were collected, and prevalence of DVA-associated CCMs was compared between these groups as well.
Statistical analysis
All data were collected and analyzed using the SAS-based statistical software package JMP12.0 (www.jmp.com). The association between age and prevalence of a CCM was studied using the Cochran-Armitage test for trends. Continuous variables were compared using a student’s t test, and categorical variables were compared with a chi-squared test. A multivariate logistic regression analysis was performed to determine if age was independently associated with DVA prevalence adjusting for all baseline characteristics. We performed two analyses, one in which age was modeled as a continuous variable and one in which age was modeled as a categorical variable (i.e., by age group).
Results
Baseline patient characteristics
A total of 1689 individuals were included. A total of 116 patients (6.9%) had CCMs associated with their DVAs and 1570 did not have CCMs. Seven CCMs (6.0%) were Zambramski Type 1, 62 (53.4%) were Type 2, 14 (12.7%) were Type 3, and 33 (28.4%) were Type 4. Mean patient age among patients with DVA-associated CCMs was 52.9 ± 17.8 compared to 46.6 ± 21.3 in the non-CCM group (P = 0.0004). Patients with DVA-associated CCMs were more likely to have DVAs in the brainstem (18 patients, 15.5% versus 51 patients, 3.2%) and basal ganglia/thalamus (7 patients, 6.0% versus 61 patients, 3.9% (P < .0001)). There was no difference in DVA-associated CCM prevalence by gender (P = 0.59). These data are summarized in Table 1.
Prevalence of cerebral cavernous malformation by age
There was a significant positive association between age and the prevalence of DVA-associated CCM (P = 0.002). The prevalence of DVA-associated CCM was 0.8% (1/122) for the 0–10 age group, 1.6% (2/127) for the 11–20 age group, 7.5% (11/146) for the 21–30 age group, 9.5% (19/200) for the 31–40 age group, 6.1% (15/245) for the 41–50 age group, 6.3% (21/336) for the 51–60 age group, 7.4% (22/297) for the 61–70 age group, and 11.6% (25/216) for the >70 age group. These data are summarized in Fig. 1. Examples of DVA-associated CCMs are provided in Figs. 2, 3, and 4.
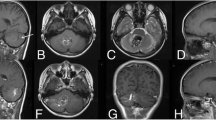
Example of cavernoma associated with DVA in a 24-year-old male. a Susceptibility-weighted imaging and b T2/FIESTA-weighted imaging demonstrate a T2 hypointense lesion in the rostral midbrain. c The lesion had intrinsic T1 hyperintensity and was associated with a draining DVA (arrow). d The patient had a cerebral angiogram which demonstrated the DVA which appeared very late in the venous phase
Example of a cavernoma associated with DVA in a 65-year-old female. a T2-weighted image shows hemosiderin staining in the left cerebellar hemisphere with a large linear flow void consistent with a DVA. b Susceptibility-weighted imaging shows marked blooming artifact consisted with a cavernoma. c and d Post-contrast T1-weighted MRI clearly demonstrates a DVA with multiple venous radicles. The cavernoma is centered in the DVA
Example of a cavernoma associated with a DVA in a 1-year-old male with staring spells. a and b Contrast-enhanced T1-weighted image demonstrating multiple venous radicles and a draining vein in the left frontal lobe. c Axial T2-weighted MRI demonstrates a cavernoma with a rim of low T2 signal and areas of T1 signal internally. d Gradient recall echo T2*-weighted image shows marked blooming artifact surrounding the cavernoma
Comorbidities and cerebral cavernous malformation
The prevalence of any seizure history in the non-CCM group was 19.1% compared to 20.7% in the CCM group (P = 0.71). The prevalence of CNS neoplasm was 7.2% in the non-CCM group compared to 5.2% in the CCM group (P = 0.41). In the non-CCM group, 4.2% of patients had a prior history of cranial radiation therapy compared to 0.9% in the CCM group (P = 0.05). These data are summarized in Table 1.
Multivariate analysis
On multivariate analysis modeling age as a continuous variable, each increasing year of age was independently associated with a higher odds of having a CCM (OR = 1.02, 95%CI = 1.01–1.03, P = 0.005). When modeled as a categorical variable (i.e., by age group), increasing age was also independently associated with a higher odds of having a CCM (P = 0.0004).
Discussion
Our study of over 1689 individuals with DVAs found a significant age-related increase in the prevalence of CCM. Less than 1% of patients in the 0–10 age group had CCM associated with their DVAs compared to almost 12% in the >70 age group. These findings suggest that (1) DVA-associated CCMs are not congenital lesions, (2) de novo CCM formation associated with DVAs is likely the rule, rather than the exception, and (3) various age-related changes in the cerebral venous system could trigger the formation of CCM associated with DVA.
A review of the literature suggests that the prevalence of CCMs among patients with DVAs increases with age; however, no study to date has directly compared prevalence across age groups. In a study of 172 younger patients with DVA, Linscott et al. found CCMs in the drainage territory of 6.2% of DVAs [12]. In a study of a predominantly adult population (mean age ~50 years), however, San Millian Ruiz et al. found that the among patients with DVAs, the prevalence of CCMs was 13.3% [8]. Other studies of CCM prevalence in the adult DVA population report prevalence rates between 3 and 40% with most larger studies demonstrating rates between 10 and 20% [7,8,9,10]. In our study of nearly 1700 patients, we found that the prevalence of CCMs in patients with DVAs was on the lower end of this spectrum with less than 5% of patients under 20 having DVA-associated CCMs compared to about 10% of older adult patients.
In general, it is thought that DVA-associated CCMs result from recurrent microhemorrhages triggering release of various growth factors that promote CCM formation a process termed hemorrhagic angiogenic proliferation [13, 14]. The trigger for microhemorrhages is generally thought to be local venous hypertension resulting from local thrombosis, stenosis, or changes in DVA angioarchitecture [14]. Sharma et al. validated the venous hypertension hypothesis when they found that CCM-associated DVAs demonstrated longer mean transit times in the draining territory of the DVA than DVAs not associated with CCMs [15]. Other studies have demonstrated that variables such as severe medullary venous tortuosity, medullary venous stenosis, or sharp angles between the radicular vein and the dominant medullary venous drainage are associated with a higher prevalence of CCMs associated with DVAs [13, 16]. It is possible that age-related changes in DVA venous angioarchitecture and propensity for a higher rate of microthrombosis with age could contribute to higher rates of CCM formation with age.
Limitations
Our study has limitations. Longitudinal data were not available for most of these patients, so we did not document the rate of de novo CCM formation. In many cases, it is difficult to distinguish between DVA-associated CCM and microhemorrhages. CCMs were identified and classified using Zambramski classification, and any Zambramski-type lesion found along the venous radicles or along the central draining portion of the DVA was included. Type 4 CCMs (i.e., microhemorrhages) occurring in isolation, defined as those identified on hemosiderin sequences only, were excluded since a microbleed due to an alternative cause (hypertensive, amyloid angiopathy, radiation, trauma) could not be excluded. However, Type 4 lesions seen within the drainage territory of the DVA without any other pattern of microbleed suggestive of another etiology were included. Ultimately, it is likely that microhemorrhages and CCM are part of a pathological continuum rather that two distinct entities. We did not analyze other clinical factors that could be associated with CCM formation including proinflammatory or pro-thrombotic states.
We must also comment on limitations with regard to imaging techniques. All images were obtained on 1.5T or 3T scanners. It is likely that scans performed at 3T are more sensitive at detecting smaller CCMs than those performed at 1.5T. There is also variability in the type of T2*-weighted imaging performed as conventional T2*-weighted gradient recall echo sequences are less sensitive at detecting microhemorrhages and CCMs than susceptibility-weighted imaging.
Conclusions
Our study demonstrated a significant, age-related increase in the prevalence of CCM associated with DVA. These findings suggest that DVA-associated CCMs are acquired lesions, possibly related to age-related changes in DVA physiology/angioarchitecture.
References
Abdulrauf SI, Kaynar MY, Awad IA (1999) A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery 44(1):41–46 discussion 46-7
Wurm G, Schnizer M, Fellner FA (2005) Cerebral cavernous malformations associated with venous anomalies: surgical considerations. Neurosurgery 57(1 Suppl):42–58 discussion 42-58
Dammann P et al (2016) Correlation of the venous angioarchitecture of multiple cerebral cavernous malformations with familial or sporadic disease: a susceptibility-weighted imaging study with 7-Tesla MRI. J Neurosurg:1–9
Cakirer S (2003) De novo formation of a cavernous malformation of the brain in the presence of a developmental venous anomaly. Clin Radiol 58(3):251–256
Campeau NG, Lane JI (2005) De novo development of a lesion with the appearance of a cavernous malformation adjacent to an existing developmental venous anomaly. AJNR Am J Neuroradiol 26(1):156–159
Desal HA et al (2005) Multiple de novo vascular malformations in relation to diffuse venous occlusive disease: a case report. Neuroradiology 47(1):38–42
Santucci GM et al (2008) Brain parenchymal signal abnormalities associated with developmental venous anomalies: detailed MR imaging assessment. AJNR Am J Neuroradiol 29(7):1317–1323
San Millan Ruiz D et al (2007) Parenchymal abnormalities associated with developmental venous anomalies. Neuroradiology 49(12):987–995
Abe T et al (1998) Coexistence of occult vascular malformations and developmental venous anomalies in the central nervous system: MR evaluation. AJNR Am J Neuroradiol 19(1):51–57
Huber G et al (1996) Regional association of developmental venous anomalies with angiographically occult vascular malformations. Eur Radiol 6(1):30–37
Zabramski JM et al (1994) The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 80(3):422–432
Linscott LL et al (2014) Brain parenchymal signal abnormalities associated with developmental venous anomalies in children and young adults. AJNR Am J Neuroradiol 35(8):1600–1607
Hong YJ et al (2010) The angioarchitectural factors of the cerebral developmental venous anomaly; can they be the causes of concurrent sporadic cavernous malformation? Neuroradiology 52(10):883–891
Perrini P, Lanzino G (2006) The association of venous developmental anomalies and cavernous malformations: pathophysiological, diagnostic, and surgical considerations. Neurosurg Focus 21(1):e5
Sharma A et al (2013) Hemodynamic effects of developmental venous anomalies with and without cavernous malformations. AJNR Am J Neuroradiol 34(9):1746–1751
Yu T et al (2016) The relation between angioarchitectural factors of developmental venous anomaly and concomitant sporadic cavernous malformation. BMC Neurol 16(1):183
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Brinjikji, W., El-Masri, A.ER., Wald, J.T. et al. Prevalence of cerebral cavernous malformations associated with developmental venous anomalies increases with age. Childs Nerv Syst 33, 1539–1543 (2017). https://doi.org/10.1007/s00381-017-3484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3484-0