Abstract
Purpose
The aim of this study was to investigate the relationship between optic nerve sheath diameter (ONSD) measurement and invasively measured intracranial pressure (ICP) in children.
Methods
ONSD measurement was performed prior to invasive measurement of ICP. The mean binocular ONSD measurement was compared to the ICP reading. Physiological variables including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse rate, temperature, respiratory rate and end tidal carbon dioxide (ETCO2) level were recorded at the time of ONSD measurement. Diagnostic accuracy analysis was performed at various ICP thresholds and repeatability, intra- and inter-observer variability, correlation between measurements in different imaging planes as well the relationship over the entire patient cohort were examined in part I of this study.
Results
Data from 174 patients were analysed. Repeatability and intra-observer variability were excellent (α = 0.97–0.99). Testing for inter-observer variability revealed good correlation (r = 0.89, p < 0.001). Imaging in the sagittal plane demonstrated a slightly better correlation with ICP (r = 0.66, p < 0.001). The ONSD measurement with the best diagnostic accuracy for detecting an ICP ≥ 20 mmHg over the entire patient cohort was 5.5 mm, sensitivity 93.2 %, specificity 74 % and odds ratio (OR) of 39.3.
Conclusion
Transorbital ultrasound measurement of the OSND is a reliable and reproducible technique, demonstrating a good relationship with ICP and high diagnostic accuracy for detecting raised ICP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The optic nerve originates from the diencephalon and is an extension of the white matter tract of the central nervous system (CNS). As the optic nerve extends into the orbital cavity, it is enveloped by a dural sleeve which is continuous with the intracranial dura mater. An increase in intracranial pressure (ICP) above a certain threshold can result in displacement of CSF from the intracranial cavity into the perineural subarachnoid space due to direct continuity between these spaces [1–4]. This increase in CSF volume within the optic nerve sheath (ONS) results in distension of the ONS and widening of its diameter. The space between the optic nerve and dural sheath is not a homogeneous space, but rather a trabeculated, septated system that likely plays a role in the CSF dynamics between the perineural space surrounding the optic nerve and the subarachnoid space of the intracranial cavity [3]. Measurement of the optic nerve sheath using transorbital ultrasound has been described as a non-invasive alternative for assessing ICP in children and adults [1, 5–8]. The use of CT and MRI to image the ONSD has also been described [9–13].
The observer variability, spatial resolution of the ultrasound probe, binocular measurement and optimal plane of measurement have been described as potential limitations of transorbital sonographic measurement of the optic nerve sheath [14–19].
The first part of this study aimed to describe the relationship between ONSD measurement and ICP over the entire patient cohort by evaluating the issues of observer reliability, plane of imaging, binocular measurement. An ICP threshold of 20 mmHg was used for the detailed analysis, and the diagnostic accuracy was then further analysed at ICP thresholds of 15, 10 and 5 mmHg.
Methods
Study design
This study was a prospective, observational analysis of a paediatric cohort of patients who had direct measurement of ICP and concurrent ultrasound measurement of the ONSD.
Reporting of data in this study was in accordance with the ‘strengthening the reporting of observational studies in epidemiology’ (STROBE) guidelines [20, 21].
Study population
Paediatric patients under the age of 14 years treated at the Red Cross War Memorial Children’s Hospital were eligible for enrolment into the study, if they were undergoing a surgical procedure for diagnostic or therapeutic purposes, and appropriate informed consent was obtained from the parents/caregivers to be enrolled into the study.
Inclusion criteria
-
(i)
Patients requiring invasive ICP monitoring based on clinical/radiological findings or undergoing a procedure where ICP could be measured safely.
Acquisition of ONS images
All ultrasound image-acquisition was performed by a single investigator, experienced in the use of ultrasound in neurosurgical patients and blinded to the invasive ICP measurement at the time of image acquisition.
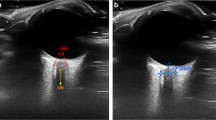
Patients were under general anaesthesia and ONSD measurement was performed when haemodynamic parameters were most stable, with patients in the supine position, head central and elevated to approximately 30°. A clear film protective dressing was placed over both eyes followed by a layer of coupling gel. Imaging of the ONS was performed using a 7–15 Mhz linear array ‘hockey stick’ ultrasound probe with the smallest available footprint (Philips, Bothell, USA) [22–24].
Three measurements were acquired in the sagittal plane followed by clockwise rotation of the probe and acquisition of three measurements in the axial plane of both eyes. The average ONSD measurement was calculated for each eye, and the mean binocular ONSD measurement was used for comparison with the invasively recorded ICP reading. All images were saved in DICOM and jpeg format.
Haemodynamic and intracranial parameters
Measurement of the ONSD was performed when the haemodynamic parameters were stable. The AF was palpated and described as open or closed. In cases where the AF was almost closed and barely palpable, it was classified as closed. The systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse rate, respiratory rate, temperature, weight and end tidal carbon dioxide (ETCO2) were all recorded at the time of ONSD measurement. Any medication given after ONSD measurement was documented. If any of these agents were given between ONSD measurement and ICP measurement and thought to affect the ICP, data from these patients were excluded from the analysis.
Measurement of ICP
All ICP measurements were recorded at the time of surgery via intraparenchymal microsensor probes or CSF pressure manometer readings during ventricular catheter placement. Particular care was taken not to lose any CSF between ventricular cannulation and connection of the manometer. The external auditory meatus was used as a reference point for ‘zeroing’ the CSF manometer readings. In cases where measurement was not possible or where the reading was not considered accurate, the data were excluded. ICP measurement was therefore a point-in-time measurement.
All ICP recordings were converted to mmHg for uniformity and to allow comparison of data with similar published studies.
Anaesthetic protocol
The goal during anaesthesia was to maintain normotension, normothermia, normonatremia, normocapnia and normovolemia. ONSD image acquisition and ICP measurement were always performed during the same anaesthesia/procedure. Minimum alveolar concentration (MAC) of the volatile agent used was maintained at a constant level between ONSD image acquisition and invasive ICP measurement. Use of positive end expiratory pressure (PEEP) and age-appropriate tidal volumes and respiratory rates were used to maintain normocarbia. Physiological parameters recorded at the time of ONSD acquisition were included in a multivariate regression model to test for effect on the relationship between ONSD and ICP.
Sample size calculation
Based on results from a local pilot study, the sample size estimate for comparison of means was calculated at 58 patients, to achieve 80 % power, with an alpha of 5 (p < 0.05). Based on the available literature, the results were expected to differ according to age category, with described age-related variation at 1 year, between 1 and 4 years and old above 4 years old. Due to the variation and inconsistency in the ages of patients at presentation, and aiming to recruit around 58 patients into each of the 3 age categories, the total sample size for the study was set at 174 patients (≤14 years old). With an estimated 10 % loss due to exclusion criteria and data loss, the study aimed to recruit 192 patients.
Statistical analysis
Data were tested for normality of distribution using the Shapiro-Wilks test. Parametric data were reported as means and standard deviations (SD) and non-parametric data reported as medians and interquartile ranges (IQR). All analyses were done using Stata IC version 12.0 (Stata Corporation LP, College Station, TX, USA) statistical software. The general association between means of numeric variables was tested by applying either Pearson’s (PCC) or Spearman’s correlation coefficient (SCC), depending on normality of data distribution. Analysis of variance (ANOVA) testing was used to determine within and between group variances for the individual ONSD readings acquired per eye.
Cronbach’s alpha statistic (α), which is a model of internal consistency, was used to test correlation and covariance for within observer testing. Bland-Altmann analysis was used to test inter-observer agreement, using limits of agreement and mean differences. Statistical significance was set at p < 0.05, and all significance testing was two sided. The independent Student’s t test was used for comparing mean values, and the Wilcoxon sum rank test/Mann–Whitney test was used to compare median values.
Results
The results in part 1 of this study include general and demographic data, testing for repeatability, observer variability, correlation between imaging planes and both eyes and an analysis of ONSD cutoff values at ICP thresholds of 20, 15, 10 and 5 mmHg over the entire patient cohort.
General and demographic data
In total, ONSD measurements were performed on 195 patients. Data from 21 patients were excluded for the following reasons: (i) haemodynamic instability, (ii) physiological manipulation required to optimise ICP, (iii) ICP-lowering agents administered between ONSD imaging and ICP measurement, (iv) incomplete datasets, (v) equipment failure, and (vi) initial ICP measurement unreliable.
Data for the remaining 174 patients were available for the final analysis. In total, 2062 out of a total of 2088 (98.8 %) ONSD measurements (acquiring 3 images in the axial plane and 3 images in the sagittal plane from each eye) were suitable for analysis.
There were 52 (30.9 %) females and 122 (69.1 %) males, with a female-to-male ratio of 0.43:1. The median age was 36 months (IQR 8–82). Fifty-six (32.2 %) children were ≤1 year old and 118 (67.8 %) >1 year old. The aetiology reflected the typical spectrum of neurological disease encountered in clinical practice and included: hydrocephalus (52.9 %), traumatic brain injury (17.2 %), tumour (with or without hydrocephalus) (9.2 %), craniosynostosis (9.2 %), cystic malformation (6.9 %), other (4.6 %) [Chiari I malformation, spinal dysraphism, effusion and macrocephaly]. Demographic details are summarised in Table 1.
Intra-operative haemodynamic parameters
Physiological parameters recorded at the time of ONSD image acquisition included: SBP, DBP, MAP, pulse rate, respiratory rate, end tidal CO2, weight and temperature. These physiological parameters were analysed in two groups, ICP < 20 mmHg and ICP ≥ 20 mmHg, with SBP (p = 0.02) and ONSD (p < 0.001) demonstrating a significant difference in the two groups (Table 2).
Length of time for ultrasound image acquisition and time between image acquisition and ICP measurement
The median length of time required for ONS image acquisition in two planes from both eyes in this study was 116 s (IQR 95–129). The recorded median time from acquisition of ONSD images to ICP measurement was 23 min (IQR 15–32 min).
Testing for repeatability of individual measurements
Measurements were repeated three times in each plane and compared using ANOVA testing and Cronbach’s alpha to determine the repeatability of these measurements. The mean ONSD measurement in the sagittal plane was 5.61 mm (SD 1) in the left eye (LESag), 5.63 mm (SD 0.99) in the right eye (RESag), and in the axial plane was 5.51 mm (SD 0.98) in the left eye (LEAx) and 5.59 mm (SD 0.96) in the right eye (REAx). Repeatability of ONSD measurements was excellent (Cronbach’s α = 0.97–0.98). These results are summarised in Table 3.
Testing for intra-observer variability
To assess the intra-observer variability, repeat measurements of the ONSD were performed on 41 randomly selected patients at a later time by the same observer, blinded to the original measurements. The mean ONSD at the first reading was 5.58 mm (SD 0.85) and at the second reading was 5.56 mm (SD 0.84) (p = 0.92). Intra-observer variability testing demonstrated excellent correlation (α = 0.99, CA).
Testing for inter-observer variability
Inter-observer variability testing was performed by comparing the mean ONSD measurements performed independently by two experienced observers in 40 patients. The mean ONSD measurement by observer 1 was 5.73 mm (SD 0.85) and by observer 2 was 5.55 mm (SD 0.87) (r = 0.89, p < 0.001). Bland-Altmann analysis revealed a mean difference between the two observers of 0.18 mm (95 % CI 0.05–0.31), with limits of agreement between −0.64 and 1 (Fig. 1).
Correlation between sagittal and axial imaging
The mean ONSD measurement in the sagittal plane (AVESag) was 5.62 mm (SD 0.96) and in the axial plane (AVEAx) was 5.56 mm (SD 0.92) (p = 0.55). Correlation between AVESag and AVEAx was excellent (r = 0.93, p < 0.001). When correlated with ICP, AVESag demonstrated a slightly better relationship (r = 0.66, p < 0.001) than AVEAx (r = 0.64, p < 0.001).
Correlation between right and left eye measurements
The mean ONSD in the right eye was 5.61 mm (SD 0.95) and in the left eye was 5.55 mm (SD 0.97). Correlation of the ONSD measurements between the two eyes was excellent (r = 0.9, p < 0.001).
Relationship between ONSD measurement and ICP over the entire cohort of patients
The relationship between ONSD and ICP over the entire cohort of patients was evaluated using the treatment threshold of 20 mmHg and then further assessed at ICP thresholds of 15, 10 and 5 mmHg to evaluate the diagnostic accuracy of ONSD measurement.
ICP dichotomised at 20 mmHg for all patients
One hundred seventy-four patients were included in this study. The median age was 36 (IQR 8–82) months. One hundred patients had an ICP < 20 mmHg, with a mean ICP of 11.68 mmHg (SD 4.5) and 74 patients had an ICP ≥ 20 mmHg, with a mean ICP of 23.87 mmHg (SD 4.33). Correlation between mean ONSD and ICP over the entire cohort was good (r = 0.66, p < 0.001, PCC) (Fig. 2).
The mean ONSD in the group with ICP < 20 mmHg was 5.08 mm (SD 0.76) and in the group with ICP ≥ 20 mmHg was 6.28 mm (SD 0.64) (p < 0.001). The optimal ONSD cutoff point for detecting an ICP ≥ 20 mmHg was 5.5 mm with a sensitivity of 93.2 %, a specificity of 74 %, PPV of 72.6 %, NPV of 93.7 %, AUROC of 0.84 (Fig. 3) and an OR of 39.3.
Applying a linear regression model to the data and controlling for age revealed that with a baseline value of 4.03 mm, for every 10 mmHg increase in ICP the ONSD increased by 0.75 mm (95 % CI 0.62–0.88, R 2 = 0.52, p < 0.001). In the group with ICP ≥ 20 mmHg, with a baseline value of 5.69 mm, this change in ONSD was 0.24 mm (95 % CI 0.09–0.5, R 2 = 0.03, p = 0.16) for every 10 mmHg increase in ICP.
In the unadjusted model, the OR for an ONSD value of 5.5 mm to detect an ICP ≥ 20 mmHg was 39.28. In the adjusted model, the OR was reduced to 26.88. Even though the OR decreased in the adjusted model, the relationship between ONSD and ICP remained statistically significant in both the unadjusted and adjusted models (Table 4).
Aetiology
Various aetiologies of raised ICP in children have been described in studies evaluating the usefulness of ONSD measurement [25–31]. Aetiology was therefore analysed to assess its effect, and the ONSD measurements were reported in each aetiological group (Table 5). The diagnostic accuracy of ONSD measurement for detecting ICP ≥ 20 mmHg in each aetiological group are summarised in Table 6. No diagnostic accuracy analysis was performed in the tumour group as all patients in this group had an ICP ≥ 20 mmHg.
ICP dichotomised at 15 mmHg for all patients
Seventy-three patients had an ICP < 15 mmHg, with a mean ICP of 9.71 mmHg (SD 3.54) and 101 patients had an ICP ≥ 15 mmHg, with a mean ICP of 22.04 mmHg (SD 4.84). The mean ONSD in the group with ICP < 15 mmHg was 4.92 mm (SD 0.8) compared to 6.08 mm (SD 0.7) (p < 0.001) in the group with ICP ≥ 15 mmHg. The optimal ONSD cutoff point for detecting ICP ≥ 15 mmHg was 5.29 mm, with a sensitivity of 88.1 %, a specificity of 78.1 % and an OR of 26.4.
ICP dichotomised at 10 mmHg for all patients
Thirty-one patients had an ICP < 10 mmHg, with a mean ICP of 6.23 mmHg (SD 2.11) and 143 patients had an ICP ≥ 10 mmHg, with a mean ICP of 19.17 mmHg (SD 6.1). The optimal ONSD cutoff point for detecting an ICP ≥ 10 mmHg was 5.04 mm, with a sensitivity of 79 %, a specificity of 74.2 % and an OR of 10.8.
ICP dichotomised at 5 mmHg for all patients
Five patients had an ICP < 5 mmHg, with a mean ICP of 3 mmHg (SD 1.4) and 169 patients had ICP ≥ 5 mmHg, with a mean ICP of 17.28 mmHg (SD 7.2). The mean ONSD in the group with ICP < 5 mmHg was 4.98 mm (SD 0.2) compared to a mean ONSD of 5.61 mm (SD 0.9) (p = 0.13) in the group with ICP ≥ 5 mmHg. The optimal ONSD cutoff point for detecting ICP ≥ 5 mmHg was 5 mm, with a sensitivity of 72.8 %, specificity of 60 % and an OR 4.01.
The diagnostic accuracy of ONSD measurement for detecting ICP at thresholds of 20, 15, 10 and 5 mmHg across the entire patient cohort are summarised in Table 7.
Discussion
The relationship between ONSD measurement and the criterion standard of invasive ICP measurement was prospectively examined in this study. The aetiology reflected the most commonly encountered paediatric neurosurgical conditions. The majority of patients included in the study had hydrocephalus (52.9 %), followed by TBI (17.2 %), with tumours and craniosynostosis, each forming 9.2 %. The concepts of repeatability and user variability remain core aspects of any suitable diagnostic modality. Specifically for transorbital ultrasonography, the most appropriate plane of imaging and the requirement for bilateral image acquisition also remain relevant questions [14, 15, 32].
Repeatability, intra-observer variability and inter-observer variability
Ballantyne et al. examined the observer variation in normal adult patients, demonstrating a median intra-observer variation of ± 0.2–0.4 mm and an inter-observer variation of ± 0.6–0.7 mm, reporting that standardisation of the technique reduced observer variability [25]. Two independent investigators demonstrated good correlation (r = 0.83) and robust agreement with mean differences close to zero between the two observers [16, 17]. In a study evaluating ONSD on MRI, a mean difference between observers of 0.11 mm was described, shown to be larger when using ultrasound, with a median inter-observer difference of 0.25 mm [32, 33]. Different ultrasound probes and imaging techniques could impact on ONSD measurement and inter-observer reliability with a reported adjusted difference of 0.21 mm between observers using a ‘standard probe’ [18].
Results from our study demonstrated excellent repeatability between each of the three ONSD measurements performed in the same plane of each eye (Cronbach’s α = 0.97–0.98). These findings were consistent in both the axial and sagittal plane. Intra-observer variability was excellent with mean ONSD values of 5.56 mm (SD 0.85) and 5.58 mm (SD 0.84), from the first and second measurements, respectively (α = 0.99). Inter-observer testing revealed a mean difference of 0.18 mm between observers. This is consistent with other described values of between 0.2 and 0.34 mm [1, 18, 30, 32, 33]. Correlation between the two observers was also very good (r = 0.89, p < 0.001). These findings confirm that transorbital ultrasound-based measurement of the ONSD yields acceptable intra- and inter-observer variability.
Correlation between imaging planes and measurements in both eyes
The ideal plane in which to measure the ONSD remains unclear. Axial images were obtained because of the ease of acquisition and the size of the ultrasound probes used. Early work by Hansen suggested that the axial plane provided more accurate measurements [34], but this was based on a relatively small patient sample and did not factor in the benefit of the smaller footprint probes available today. As the ONS is not a perfect sphere, differences in the measurements acquired in various planes are likely [35, 36].
Evaluating the optimal plane for measurement, i.e. sagittal vs axial, demonstrated a slight advantage in the sagittal plane over the axial plane (r = 0.66 vs r = 0.64). This difference was minimal, suggesting that measurement in either plane was an acceptable option. Our study allowed adequate imaging in the sagittal plane largely because of the small footprint, high frequency probe used, differentiating it from most other studies using probes with a larger footprint [26, 29, 30, 34, 35, 37].
Differences between measurements obtained in the left and right eye were analysed and the results demonstrated a good correlation between ONSD values from both eyes (r = 0.9, p < 0.001). The benefit of using the mean value of ONSD measurements from both eyes however, remains a logical option based on described individual anatomical variation and the possibility of unilateral pathology [34, 38]. Given the findings of this study and the excellent correlation demonstrated between the three measurements, if the imaging quality is considered adequate, it may be acceptable to perform two measurements in each eye, in either the sagittal or axial plane (depending on the probe shape and size), and use the mean ONSD measurement for estimating ICP.
Analysis over the entire cohort
The linear relationship between ONSD and ICP and recommendation of the technique as a simple bedside method for non-invasively assessing ICP, has been widely described [8, 15, 26, 32, 33, 39, 40]. The diagnostic accuracy for detecting raised ICP and the optimal cutoff values however, are still unclear, particularly in children [8, 27, 41].
Specific limitations of this technique have hampered its widespread use; these include poor specificity, variation in the recommended ONSD cutoff values for predicting raised ICP, inter-rater and inter-individual variability and limited understanding of elastic nature of the ONS [7, 14, 25, 42, 43]. The recommended ONSD cutoff value in adults ranges widely from 4.1 to 5.9 mm [6, 32, 33, 39, 44, 45]. In children, this is further complicated by age-related upper limits of ONSD measurement [1, 25, 26, 28, 31].
Evaluating the entire patient cohort in part I of this study demonstrated a mean ONSD in the group with ICP < 20 mmHg of 5.08 mm (SD 0.76) and in the group with ICP ≥ 20 mmHg of 6.28 mm (SD 0.64) (p < 0.001). The optimal ONSD cutoff point in this study for detecting ICP ≥ 20 mmHg across the entire patient cohort was 5.5 mm with a sensitivity of 93.2 %, a specificity of 74 % and an OR of 39.3. In a multivariate model adjusting for appropriate confounding variables, the OR was adjusted to 26.9 (Table 4), suggesting that the likelihood of detecting an ONSD measurement > 5.5 mm is 26.9 times higher in children with an ICP ≥ 20 mmHg than in children with an ICP < 20 mmHg. In a recent systematic review, Dubourg at al describe an even higher pooled diagnostic OR of 51 and recommended that a high sensitivity be favoured over a high specificity when using sonographic ONSD measurement to estimate ICP [8]. It is worth noting that this review only included work done in adults.
With a baseline diameter of 4.03 mm, our study described a change in ONSD of 0.75 mm (95 % CI 0.62–0.88, R 2 = 0.52, p < 0.001), after controlling for age as a confounding variable, for every 10 mmHg change in ICP. This is useful in describing the relationship between ONS distension and increase in ICP. This relationship was weaker when ICP was ≥20 mmHg, i.e. 0.24 mm (95 % CI 0.09–0.5, R 2 = 0.03, p = 0.16) for every 10 mmHg change in ICP, suggesting a variation in the response of the ONS at different ICP thresholds. This is consistent with experimental data reporting that ONSD distensibility differed at certain ICP thresholds and likely approached a maximum distensibility at higher ICP values [34, 42]. The described change in ONSD was 0.25 mm for every 10 mmHg increase in ICP in the Hansen study, but no baseline diameter was described [34].
The findings of our study underscore the need to investigate the elasticity of the ONS as a marker of its stiffness or distensibility in order to improve our understanding of the ONS response to change in ICP [42, 43].
The higher cutoff values detected for children with craniosynostosis (6.17 mm) and lower cutoff values with intracranial cysts (4.5 mm) suggest that aetiology may have a role to play in determining the baseline ONSD and the response to an increase in ICP. While the mean ICP in these two groups was also significantly different (p = 0.047), this finding does raise the concern that ONSD cutoff recommendations should take aetiology into account.
The ONSD cutoff values with the best diagnostic accuracy for ICP ≥ 15 mmHg, ≥10 mmHg and ≥5 mmHg described in this study were 5.3, 5.04 and 5 mm, respectively (Table 7). The diagnostic accuracy of ONSD was better for detecting an ICP ≥ 20 mmHg than it was for and ICP ≥ 5 mmHg (OR of 39.5 vs. 4.01). The paediatric literature evaluating ONSD measurement suggests that age is very likely a confounding factor [1, 25, 26, 29]. For this reason, the historically described age thresholds of 1 year [25, 26] and 4 years [1, 27] were used to subcategorise the data for further analysis and comparison to published data. These results as well as an analysis of patency of the anterior fontanelle are presented in part II of this work.
Conclusion
Transorbital sonographic measurement of the ONSD is a reliable and repeatable technique, demonstrating a good relationship with ICP, particularly at higher thresholds.
References
Helmke K, Hansen HC (1996) Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. Ped Radiol 26(10):701–705
Hansen HC, Helmke K (1996) The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat 18(4):323–328
Killer HE, Laeng HR, Flammer J, Groscurth P (2003) Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalm 87(6):777–781
Hayreh SS (1984) The sheath of the optic nerve. Ophthalmologica 189(1–2):54–63
Geeraerts T, Launey Y, Martin L et al (2007) Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Int Care Med 33(10):1704–1711
Kimberly HH, Shah S, Marill K, Noble V (2008) Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med 15(2):201–204
Tsung JW, Blaivas M, Cooper A, Levick NR (2005) A rapid noninvasive method of detecting elevated intracranial pressure using bedside ocular ultrasound: application to 3 cases of head trauma in the pediatric emergency department. Ped Emerg Care 21(2):94–99
Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B (2011) Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Int Care Med 37(7):1059–1068
Steinborn M, Fiegler J, Kraus V et al (2011) High resolution ultrasound and magnetic resonance imaging of the optic nerve and the optic nerve sheath: anatomic correlation and clinical importance. Ultraschall Med 32(6):608–613
Singhal A, Yang MM, Sargent MA, Cochrane DD (2013) Does optic nerve sheath diameter on MRI decrease with clinically improved pediatric hydrocephalus? Child Nerv Syst 29(2):269–274
Padayachy LC, Kilborn T, Carrara H, Figaji AA, Fieggen GA (2015) Change in optic nerve sheath diameter as a radiological marker of outcome from endoscopic third ventriculostomy in children. Child Nerv Syst 31(5):721–728
Watanabe A, Kinouchi H, Horikoshi T, Uchida M, Ishigame K (2008) Effect of intracranial pressure on the diameter of the optic nerve sheath. J Neurosurg 109(2):255–258
Legrand A, Jeanjean P, Delanghe F, Peltier J, Lecat B, Dupont H (2013) Estimation of optic nerve sheath diameter on an initial brain computed tomography scan can contribute prognostic information in traumatic brain injury patients. Crit Care 17(2):R61
Potgieter DW, Kippin A, Ngu F, McKean C (2011) Can accurate ultrasonographic measurement of the optic nerve sheath diameter (a non-invasive measure of intracranial pressure) be taught to novice operators in a single training session? Anaesth Int Care 39:95–100
Bäuerle J, Nedelmann M (2011) Sonographic assessment of the optic nerve sheath in idiopathic intracranial hypertension. J Neurol 258(11):2014–2019
Bäuerle J, Schuchardt F, Schroeder L, Egger K, Weigel M, Harloff A (2013) Reproducibility and accuracy of optic nerve sheath diameter assessment using ultrasound compared to magnetic resonance imaging. BMC Neurol 13(1):187
Bäuerle J, Lochner P, Kaps M, Nedelmann M (2012) Intra‐ and inter-observer reliability of sonographic assessment of the optic nerve sheath diameter in healthy adults. J Neuroimaging 22(1):42–45
Shah S, Kimberly H, Marill K, Noble VE (2009) Ultrasound techniques to measure the optic nerve sheath: is a specialized probe necessary? Med Sci Monitor 15(5):MT63–MT68
Ballantyne SA, O’Neill G, Hamilton R, Hollman AS (2002) Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound 15(3):145–149
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) Strobe Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med 45(4):247–251
Vandenbroucke JP, Von Elm E et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Int Med 147(8):W-163
Lizzi FL, Mortimer AJ (1988) Bioeffects considerations for the safety of diagnostic ultrasound–preface. J Ultrasound Med 7(9):S1–S38
Nelson TR, Fowlkes JB, Abramowicz JS, Church CC (2009) Ultrasound biosafety considerations for the practising sonographer and sonologist. J Ultrasound Med 28:139–150
European Federation for Societies of Ultrasound in Medicine and Biology (EFSUMB) (1998) Clinical safety statement for diagnostic ultrasound safety. Tours, France. 1998. Eur J Ultrasound 8:67–68
Ballantyne J, Hollman A, Hamilton R et al (1999) Transorbital optic nerve sheath ultrasonography in normal children. Clin Rad 54(11):740–742
Newman WD, Hollman AS, Dutton GN, Carachi R (2002) Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalm 86(10):1109–1113
Moretti R, Pizzi B (2011) Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesth Scand 55(6):644–652
Malayeri AA, Bavarian S, Mehdizadeh M (2005) Sonographic evaluation of optic nerve diameter in children with raised intracranial pressure. J Ultrasound Med 24(2):143–147
Beare NA, Kampondeni S, Glover SJ et al (2008) Detection of raised intracranial pressure by ultrasound measurement of optic nerve sheath diameter in African children. Trop Med Int Health 13(11):1400–1404
Steinborn M, Friedmann M, Hahn H et al (2015) Normal values for transbulbar sonography and magnetic resonance imaging of the optic nerve sheath diameter (ONSD) in children and adolescents. Ultraschall Med 36(1):54–58
Driessen C, Eveleens J, Bleyen I, Van Veelen ML, Joosten K, Mathiijssen I (2014) Optical coherence tomography: a quantitative tool to screen for papilledema in craniosynostosis. Childs Nerv Syst 30:1067–1073
Moretti R, Pizzi B, Cassini F, Vivaldi N (2009) Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care 11(3):406–410
Geeraerts T, Duranteau J, Benhamou D (2008) Ocular sonography in patients with raised intracranial pressure: the papilloedema revisited. Crit Care 12(3):150
Hansen HC, Helmke K (1997) Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg 87(1):34–40
Blehar DJ, Gaspari RJ, Montoya A, Calderon R (2008) Correlation of visual axis and coronal axis measurements of the optic nerve sheath diameter. J Ultrasound Med 27(3):407–411
Geeraerts T, Bergès O, Merceron S et al (2009) Reply to Copetti and Cattarossi. Int Care Med 35(8):1490–1491
McAuley D, Paterson A, Sweeney L (2009) Optic nerve sheath ultrasound in the assessment of paediatric hydrocephalus. Child Nerv Syst 25(1):87–90
Hayreh SS (1964) Pathogenesis of oedema of the optic disc (papilloedema): a preliminary report. Br J Ophthalm 48(10):522
Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL (2011) Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care 15(3):506–515
Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M (2007) Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med 49(4):508–514
Strumwasser A, Kwan RO, Yeung L et al (2011) Sonographic optic nerve sheath diameter as an estimate of intracranial pressure in adult trauma. J Surg Res 170(2):265–271
Hansen HC, Lagreze W, Krueger O, Helmke K (2011) Dependence of the optic nerve sheath diameter on acutely applied subarachnoidal pressure—an experimental ultrasound study. Acta Ophthalm 89(6):e528–e532
Padayachy LC, Fieggen AG (2015) Optic nerve sheath diameter as criteria for endoscopic third ventriculostomy failure in children. Child Nerv Syst 31(8):1219
Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, Karabinis A (2008) Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care 12(3):R67
Wang L, Feng L, Yao Y et al (2015) Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PLoS One 10(2):e0117939
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Rights and permissions
About this article
Cite this article
Padayachy, L.C., Padayachy, V., Galal, U. et al. The relationship between transorbital ultrasound measurement of the optic nerve sheath diameter (ONSD) and invasively measured ICP in children. Childs Nerv Syst 32, 1769–1778 (2016). https://doi.org/10.1007/s00381-016-3067-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-016-3067-5