Abstract
Desmoplastic infantile ganglioglioma (DIG) and supratentorial giant cerebral aneurysm are each extremely rare entities in infants. Here, we present the case of an 8-day old boy who had both of these conditions concurrently. To our knowledge, there is no previous case reported of a patient with coexisting DIG and giant aneurysm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant brain tumors in neonates are a surgical and therapeutic challenge. Since chemotherapy and radiation are not well tolerated in this age group, surgical resection may be the only option. However, these tumors tend to be large and very vascular making surgical excision difficult. The case we report highlights these challenges and the need for a second look surgery even when the initial histopathology suggested a malignant lesion. The decision-making was further complicated by presence of what was thought to be a small venous aneurysm on MR and finally diagnosed to be giant arterial aneurysm in association with the tumor.
The incidence of both tumor and aneurysm coexisting is approximately 1 % [1–4] with modern imaging techniques. However, it is still uncommon to encounter both an aneurysm and primary brain tumor in practice. We performed a literature search using the term “desmoplastic infantile ganglioglioma” in PubMed and resulted in 112 pieces of literature reporting DIG and its associated findings, diagnostic characteristics, and histopathological findings, but none have reported a giant aneurysm associated with this lesion. In this case report, we discussed the unique imaging findings, the clinical management, etiology and treatment challenges and considerations for this particular patient, and the pathophysiology behind the formation of concurrent aneurysms and management considerations.
Case report
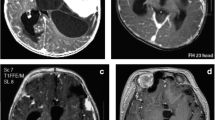
The patient initially presented as a transfer to our tertiary care facility at 8 days of life for a large intracranial mass. He was born full term with a head circumference at 97th percentile and a tight full fontanelle. He had no obvious motor deficit. An MRI was performed at an outside facility and demonstrated a large right fronto-parieto-temporal lesion, with complex heterogenous enhancement pattern with associated areas of hemorrhage, prominent vascular channels, and a possible venous aneurysm (Fig. 1). Based on the MRI findings, it was felt that this lesion could be a high-grade neoplasm. He was taken to the operating room for large fronto-tempo-parietal craniotomy with an intention of resection if the histopathology was suggestive of a low grade tumor. The tumor was encountered very near the surface of the brain. Resection was started laterally in the temporal region. The tumor was extremely vascular, and after a sizeable specimen was removed, hemostasis was achieved with difficulty. Intraoperative frozen section was consistent with high-grade astrocytoma. It was, therefore, decided to not proceed with resection and instead plan for chemotherapy. This decision was made based on the size, vascularity, and pathology of the tumor. An external ventricular drain was also placed at the time of this surgery and subsequently internalized as a shunt.
The permanent section confirmed the frozen section diagnosis of a high grade astrocytoma (Fig. 2). He was assessed by the oncology service and underwent six cycles of chemotherapy.
Pathology slides from first surgery showed characteristics of high-grade glioma. Specimen has high cellularity, most of the cells stains positive for GFAP with a relatively high Ki-67 proliferation index (25–30 %). There is no connective tissue, and all of the neuronal marker (Neu, Synaptophysin and NFP) are negative. CD34 shows abundant capillaries but no staining in tumor cells
The patient did quite well with chemotherapy. He continued to grow and meet normal developmental milestones. He had regular follow-up in our clinic, and his parents were interested in pursuing aggressive surgical treatment. A follow-up MRI at 6 months of age revealed stable size of the tumor and increased size of a medially located rather well-defined lesion which had initially been felt to be an associated venous aneurysm (Fig. 3). A subsequent MR showed further increase in the size of the aneurysm, and time of flight imaging this time suggested an arterial aneurysm. The risks and benefits of pursuing resection of both lesions were discussed at length with the family as well as in the multidisciplinary neuro-oncology tumor board, and it was decided to proceed with surgical resection. Preoperative angiogram was planned for delineation of the aneurysm and possible embolization, as well as embolization of this extremely vascular tumor.
The tumor was fed from both internal and external carotid arteries. The external carotid component from a branch of the middle meningeal artery was successfully embolized. The aneurysm was located on a distal frontal branch of the anterior cerebral artery and filled from injections to ACA and MCA precluding embolization (Fig. 4).
Resection was carried out in two stages. First, a large right fronto-parieto-temporal craniotomy was performed utilizing the same bone flap that was fashioned in the initial surgery. The tumor was readily identified on the surface. A thick-walled aneurysm was located between the tumor and the falx in the interhemispheric fissure. Intraoperative doppler ultrasound was used to confirm the presence of blood flow to the aneurysm. The feeding branch from the ACA was identified. The aneurysm was located on the lateral side of the vessel with a very wide neck. Since the distal part of the vessel seemed to be supplying the tumour, it was coagulated and cut proximal to the aneurysm. Ultrasound was used to confirm cessation of flow to the aneurysm after securing the feeding vessels.
Subsequent circumferential dissection was performed, and roughly three quarters of tumor was safely resected (Fig. 5). By this time, the patient had required replacement of three-quarters of his total blood volume. It was therefore decided to delay the remainder of the resection to a second stage several days later. Meticulous hemostasis was achieved, the bone flap replaced, and scalp re-approximated. The patient was transferred to the intensive care unit.
The patient did well postoperatively. He was medically optimized and recovered well from his initial operation. He returned to the operating room several days later for second stage resection of residual tumor. A gross total resection was achieved without any intraoperative complications (Fig. 6). The patient recovered well without any permanent neurologic deficits. Final pathology was consistent with desmoplastic infantile ganglioglioma and giant arterial aneurysm (Fig. 7).
a Microscopic examination of specimen from second surgery showed a generally cellular tumor. Special stains reviewed the tumor is distinctly biphasic with a network of cellular and collagenous tissue, highlighted with masson trichrome and reticulin stains, outlining innumerable smaller collagen-free nests of neuroglial tissue of varying cellularity. Ki-67 proliferation index labeled 5–7 % of cells. GFAP stain showed numerous fibrillated cells in the nests and sometimes lining the nests. S100 showed the rounded glial cells and scattered cells in the nests. The neuroglial nests showed diffuse immunoreactivity to synaptophysin and neurofilament protein. b Section from the aneurysm wall (hematoxylin and eosin stain. ×200 original magnification) shows well formed fibrous wall continuous with adventitia. No smooth muscle media or elastic fibers are seen
Discussion
Although there are no reports of coexisting DIG and giant aneurysm, cerebral aneurysms has been associated with a wide variety of tumors such as meningioma, glioma, pituitary adenoma, craniopharygioma, lymphoma, dermoid tumor, epidermoid tumor, choroid plexus adenoma, and chordoma [3, 5–9]. Patients may experience symptoms from the tumor (54–78 %), or aneurysm rupture (17–45 %), or both in 6 % of cases [3, 6, 10]. In fact, most cases of concurrent aneurysm and tumor are spatially separated and often one entity is incidentally discovered on imaging for the other [11]. Though speculative, the concurrent findings of both tumor and aneurysm suggest a causal relationship. The pathophysiology of these coexisting lesions is unclear, but several hypotheses have been described. One hypothesis is that the vascularity of the tumor creates a high-flow phenomenon and induces changes in the arterial wall resulting in aneurysm formation and subsequent enlargement [3, 12]. In addition, the development of the anterior circulation branches was likely abnormal in our patient as they were displaced by the large tumor mass. Perhaps a vascular confluence was created, the interface of which was similar to a vascular bifurcation and therefore susceptible to aneurysm formation. Although not in our case, there are reported cases of tumor invasion into the aneurysm wall, which point towards a relationship between tumor growth and aneurysm formation and subsequent enlargement [7, 13, 14]. This is primarily seen in glial tumors, lymphoma, and pituitary tumors [7, 14–16]. Genetic factors can also predispose an individual to developing congenital tumors [3]. Growth hormone can also promote the development of aneurysm in some tumors such as pituitary adenoma [17]. Moreover, there are also reports of traumatic aneurysms caused by intracranial tumor surgery [18, 19]. Another possibility is the formation of a pseudoaneurysm from the pressure exerted by the tumor; however, the pathologic section showed all three layers of the vessel wall to be intact, with no extraluminal hematoma, which would exclude a diagnosis of pseudoaneurysm.
Treatment of concurrent brain tumor and cerebral aneurysms can be a challenge, and is based on symptoms produced by the lesion, nature of tumor, and location of tumor and aneurysm. Spontaneous hemorrhage inside the tumor or in the surrounding brain has been reported in high-grade glial tumors while spontaneous subarachnoid hemorrhage is not common [20]. The risk of rupture aneurysm also needs to be considered and is well-documented with multiple randomized, controlled trials [21–25]. The 5-year risk of rupture for giant aneurysm approaches 40 % in anterior circulation and certain morphologies such as having a bleb can increase the rupture risk to 28.3 %/year [23, 26]. Although the data in pediatric population is not as well categorized, one study reports the annual risk of rupture of aneurysms is 0.4 %/year in children, and the highest risk factor is previous subarachnoid hemorrhage [27]. The morbidity of treating unruptured aneurysms depends on size and characteristics of aneurysm, as well as methods of treatment (open vs. endovascular treatment) but ranges anywhere from 2–11 % [23, 26]. Therefore, treatment decision must balance the natural risk of aneurysm rupture, risk of treatment, with the best scenario being clipping of aneurysm and resection of lesion with the same approach. Endovascular intervention should also be employed as either a primary treatment option or adjunctive treatment option for the aneurysm and/or embolization of the tumor pre-operatively. Table 1 included some previously reported cases of double pathology and their management options [6, 11, 28].
The pediatric population carries its unique challenges as well. Brain tumors that present in infancy often tend to be large and vascular, and the limited blood volume of the patient makes their excision challenging. Preoperative embolization at this age carries its own risks and is limited by the size of the vessels. Often for these reasons excision may need to be staged. Management plan in this patient was further complicated by the initial histopathology suggestive of a high grade lesion. While the specimen from the first surgery was sizeable, a similar initial interpretation of malignant lesion in a DIG has been reported previously [29].
This case highlights the potential complexity of large brain tumors in infants. The associated finding of a giant aneurysm in relation to the DIG is rare and not reported previously. Treatment considerations need to incorporate lesions, the tumor and the associated aneurysm and each carries independent risks and morbidities of treatment. In addition, it is important to recognize the variability of histologic and radiological features of DIG to avoid misdiagnosis on imaging and histopathology. Furthermore, this case illustrates the importance of considering a multi-stage resection in the case of a large, complicated, and vascular pediatric tumor.
References
Brzeziński J, Kotwica Z, Polis Z (1989) Brain tumors associated with intracranial vascular anomalies. Zentralblatt Für Neurochir 50:201–202
Handa J, Matsuda I, Handa H (1976) Association of brain tumor and intracranial aneurysms. Surg Neurol 6:25–29
Pia HW, Obrador S, Martin JG (1972) Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien) 27:189–204
Vernooij MW, Ikram MA, Tanghe HL et al (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357:1821–1828. doi:10.1056/NEJMoa070972
Goodman ML, Nelson PB (1988) Association of an epidermoid tumor with an aneurysm of the anterior communicating artery. Neurosurgery 23:392–395
Licata C, Pasqualin A, Freschini A et al (1986) Management of associated primary cerebral neoplasms and vascular malformations: 1. Intracranial Aneurysms. Acta Neurochir (Wien) 82:28–38
Roitberg BZ, Cochran EJ, Thornton J, Charbel FT (2000) Giant anterior communicating artery aneurysm infiltrated with a primary cerebral lymphoma: case report. Neurosurgery 47:458–462
Shigemori M, Tokunaga T, Miyagi J et al (1991) Multiple brain tumors of different cell types with an unruptured cerebral aneurysm—case report. Neurol Med Chir (Tokyo) 31:96–99
Terasaki M, Abe T, Tajima Y et al (2006) Primary choroid plexus T-cell lymphoma and multiple aneurysms in the CNS. Leuk Lymphoma 47:1680–1682. doi:10.1080/10428190600612503
Scamoni C, Dorizzi A, Dario A et al (1997) Intracranial meningioma associated with cerebral artery aneurysm. Report of two cases and review of the literature. J Neurosurg Sci 41:273–281
Suslu HT, Bozbuga M (2011) Primary brain tumors associated with cerebral aneurysm: report of three cases. Turk Neurosurg 21:216–221. doi:10.5137/1019-5149.JTN.2487-09.1
Delfini R, Domenicucci M, Ferrari M (1990) Association of intracranial meningiomas and aneurysms. Report of three cases and review of the literature. J Neurosurg Sci 34:51–56
Andrews BT, Raffel C, Rosegay H (1985) Subarachnoid hemorrhage from a peripheral intracranial aneurysm associated with malignant glioma: report of a case. Neurosurgery 17:645–649
Mangiardi JR, Aleksic SN, Lifshitz M et al (1983) Coincidental pituitary adenoma and cerebral aneurysm with pathological findings. Surg Neurol 19:38–41
Aichholzer M, Gruber A, Haberler C et al (2001) Intracranial hemorrhage from an aneurysm encased in a pilocytic astrocytoma—case report and review of the literature. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 17:173–178. doi:10.1007/s003810000364
Kung PC, Lee JC, Bakay L (1969) Vascular invasion by glioma cells in man: an electron microscopic study. J Neurosurg 31:339–345. doi:10.3171/jns.1969.31.3.0339
Pant B, Arita K, Kurisu K et al (1997) Incidence of intracranial aneurysm associated with pituitary adenoma. Neurosurg Rev 20:13–17
Dunn IF, Woodworth GF, Siddiqui AH et al (2007) Traumatic pericallosal artery aneurysm: a rare complication of transcallosal surgery. Case report. J Neurosurg 106:153–157. doi:10.3171/ped.2007.106.2.153
Taylor PE (1961) Delayed postoperative hemorrhage from intracranial aneurysm after craniotomy for tumor. Neurology 11:225–231
Iwama T, Ohkuma A, Miwa Y et al (1992) Brain tumors manifesting as intracranial hemorrhage. Neurol Med Chir (Tokyo) 32:130–135
Molyneux AJ, Kerr RSC, Yu L-M et al (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817. doi:10.1016/S0140-6736(05)67214-5
(1998) Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 339:1725–1733. doi:10.1056/NEJM199812103392401
Wiebers DO, Whisnant JP, Huston J et al (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Rinkel GJ, Djibuti M, Algra A, van Gijn J (1998) Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke J Cereb Circ 29:251–256
Wardlaw JM, White PM (2000) The detection and management of unruptured intracranial aneurysms. Brain J Neurol 123(Pt 2):205–221
Tsukahara T, Murakami N, Sakurai Y et al (2005) Treatment of unruptured cerebral aneurysms; a multi-center study at Japanese national hospitals. Acta Neurochir Suppl 94:77–85
Koroknay-Pál P, Niemelä M, Lehto H et al (2013) De novo and recurrent aneurysms in pediatric patients with cerebral aneurysms. Stroke 44:1436–1439. doi:10.1161/STROKEAHA.111.676601
Gökalp HZ, Avman N, Ozkal E, Gökben B (1980) Brain tumour associated with intracranial arterial aneurysm. Acta Neurochir (Wien) 53:267–273
Duffner PK, Burger PC, Cohen ME et al (1994) Desmoplastic infantile gangliogliomas: an approach to therapy. Neurosurgery 34:583–589, discussion 589
Conflict of interest
All authors have no conflict of interest.
Funding
This study is funded by Providence Hospital and Medical Center Department of Medical Education.
Informed consent
Informed Consents were obtained from guardian of patient described in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
To, C.Y., Rajah, G., Klein, E. et al. Desmoplastic infantile ganglioglioma with associated giant aneurysm—case report. Childs Nerv Syst 31, 1413–1418 (2015). https://doi.org/10.1007/s00381-015-2722-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2722-6