Abstract
Background
Left ventricular (LV) global longitudinal strain (GLS) has emerged as a more sensitive index than LV ejection fraction (LVEF) for detecting subclinical LV dysfunction. We examined whether changes in GLS values are associated with the long-term prognosis of patients with a preserved LVEF and acute decompensated heart failure (HF).
Methods
We studied 100 consecutive patients (mean age: 71 years) who were hospitalized for HF with preserved ejection fraction (HFpEF) and had a preserved LVEF (≥ 50%) in both the acute and stable phases. We performed two-dimensional speckle-tracking echocardiography in the acute (GLS-acute) and stable (GLS-stable) phases at a median of 2 and 347 days after admission, respectively, and calculated the rate of change of the absolute value of GLS-stable with respect to that of GLS-acute. An improved GLS was defined as a rate of change in GLS ≥ 16%, and a non-improved GLS was a rate of change < 16%. The primary endpoint was the occurrence of major cardiovascular events (MACE).
Results
During a mean follow-up period of 1218 days, MACE occurred in 26 patients, including 8 all-cause deaths and 18 readmissions for HF. The rate of change in GLS for patients with MACE was lower than compared to those without MACE (10.6% vs 26.0%, p < 0.001). Multivariate Cox regression analyses indicated the rate of change in GLS was an independent predictor of MACE (p < 0.001). A non-improved GLS was correlated with a high risk of MACE.
Conclusion
Changes in GLS values could be useful for the long-term risk stratification of patients hospitalized for HFpEF and persistently preserved LVEF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) comprises about half of all HF hospitalizations [1]. Furthermore, the prognosis of patients with HFpEF is reported to be as poor as that of patients with HF with reduced ejection fraction (HFrEF) [2, 3]. An assessment of the left ventricular (LV) global longitudinal strain (GLS) has emerged as a more sensitive modality than an evaluation of the LV ejection fraction (LVEF) to quantify LV contractile performance for HFpEF [1, 4] and has a greater prognostic value compared to LVEF [5]. An abnormal GLS on admission was associated with poor short-term outcomes in patients with HFpEF and acute decompensated HF [4]. GLS in the chronic setting was an independent predictor of HF-related hospitalization and cardiovascular death in patients with HFpEF [6, 7]. However, the prognostic value of a change in GLS among patients with HFpEF remains unclear. The purpose of this study was to investigate the association of changes in GLS values with the long-term prognosis of patients with HFpEF and acute decompensated HF.
Materials and methods
Study design
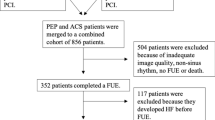
Of 2346 patients who were hospitalized for worsening HF from January 2013 through April 2021 at Fujita Health University Coronary Care Unit, 172 patients had a preserved LVEF (≥ 50%) in both the acute and stable phases. We excluded 72 patients who had any one of the following: atrial fibrillation (AF), severe valvular heart disease, an inadequate echocardiographic image quality for strain analyses, or undergoing dialysis. A total of 100 patients were included in the final analysis (Fig. 1). HF on admission was diagnosed according to the Framingham criteria [8]. Physicians independently selected the appropriate therapy and managed the patients following standard protocols using outcome measurements, such as an improvement in symptoms, physical examination findings, pulmonary congestion on chest radiographs, and echocardiographic findings. We performed speckle-tracking echocardiography and measurements of serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels on the same day in the acute phase (interval between admission and echocardiography; median, 2 days [interquartile range (IQR), 1–2 days]) and in the stable phase (interval between admission and echocardiography; median, 347 days [IQR, 51–872 days]). All patients were clinically stable (i.e., unchanged New York Heart Association [NYHA] functional class, no significant changes in the hemodynamic status, and receiving medical therapy) in the stable phase. The primary endpoint was the occurrence of major cardiovascular events (MACE): all-cause death and readmission for HF. The ethics committee of Fujita Health University approved this study (study protocol number HM19-230), which was conducted in accordance with the Declaration of Helsinki. All patients individually provided written informed consent.
Echocardiographic analysis
An echocardiographic examination was performed using the commercially available Vivid 7, Vivid E9 or Vivid E95 systems (GE Vingmed, Horton, Norway). All data were stored digitally for off-line analysis on EchoPAC PC (GE Vingmed, Horton, Norway).
Standard echocardiographic measurements were obtained using two-dimensional (2D) and Doppler measurements, in accordance with the American Society of Echocardiography guidelines [9, 10]. LVEF and left atrial volume were calculated by the biplane Simpson’s method from apical 4- and 2-chamber views.
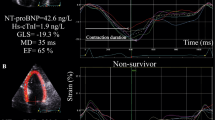
Longitudinal strain was assessed from the three apical views (4-, 2-, and 3-chamber). For speckle tracking, endocardial border was manually traced at end-systole, and the integrity was visually confirmed. In the cases of insufficient tracking, manual correction of the endocardial tracking was attempted; if the results were still unsatisfactory, then the entire study was excluded from the analysis. GLS was obtained by averaging the peak strain values from the 17 regional longitudinal strains. Peak GLS was computed automatically. All analyses were performed by a single experienced operator blinded to other patient characteristics and outcomes.
Study variables and definitions
Because GLS is a negative value, we adopted the absolute value |X| for a simpler interpretation. We calculated the rate of change of the absolute value of GLS in the stable phase (GLS-stable) with respect to that in the acute phase (GLS-acute). Patients were divided into two groups based on the rate of change in GLS: (1) improved GLS, a rate of change in GLS ≥ 16%; and (2) non-improved GLS, a rate of change in GLS < 16%. We calculated the serum creatinine-based estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease Study equation, as recommended by the Japan Chronic Kidney Disease Initiative [11].
Statistical methods
JMP version Pro 15 (SAS Institute Inc., Cary, NC, USA) software was used for all statistical analyses. Data are presented as number and frequency for categorical variables and mean ± SD or median with IQR for continuous variables. Considering that the serum NT-proBNP data were irregularly distributed, analyses were performed after log transformation to meet the criteria for use in normalized statistical approaches, after statistical confirmation. The clinical and echocardiographic characteristics were compared by chi-square analysis for categorical variables and by Mann–Whitney U test and Student’s t test for continuous variables. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off values of continuous variables. Kaplan–Meier curves were plotted and compared using the log-rank test. Hazard ratios and 95% confidence intervals were calculated for each factor via the Cox proportional hazards analysis. All baseline variables with p < 0.05 in univariate analyses were integrated into the Cox multivariate model to determine the independent predictors of MACE. A p-value < 0.05 was considered significant.
Results
We enrolled 100 patients with a mean age of 71 years (22–94 years). Baseline patient characteristics are summarized in Table 1. During a mean follow-up period of 1218 days, MACE occurred in 26 patients (26%), including 8 all-cause deaths and 18 readmissions for HF. Patients who experienced MACE had higher levels of NT-proBNP in the stable phase, and lower levels of hemoglobin in the stable phase and eGFR in the acute and stable phases. Between patients with and without MACE, there were no significant differences in the systolic blood pressure and heart rate in the acute and stable phases. In addition, there were no significant differences in medications at discharge between the two groups. Echocardiographic parameters are presented in Table 2. Compared to patients without MACE, those with MACE had lower levels of GLS-stable. The rate of change in GLS for patients who experienced MACE was lower than that for those who did not (10.6% vs 26.0%, p < 0.001).
Univariate Cox regression analyses showed that the NT-proBNP level in the stable phase, GLS-stable, eGFR in the stable phase, and the rate of change in GLS were significant predictors of MACE, while LVEF in the acute and stable phases, the NT-proBNP level in the acute phase, and GLS-acute were not significant predictors of MACE. All baseline variables with p < 0.05 in univariate analyses were integrated into the Cox multivariate model. As revealed in the Cox multivariate analysis, the rate of change in GLS, eGFR in the stable phase, and the NT-proBNP level in the stable phase were significant independent predictors of MACE (Table 3). Patients with a non-improved GLS had a higher risk of MACE compared to those with an improved GLS (p < 0.001; Fig. 2). In ROC analyses, the optimal cut-off values of a rate of change in GLS for predicting MACE, determined as the level with the largest sum of sensitivity plus specificity, were 16% (sensitivity 0.77, specificity 0.74, AUC 0.81) (Fig. 3).
Kaplan–Meier curves for MACE according to the rate of change in GLS. An improved GLS indicates the rate of change of the absolute value of GLS in the stable phase (GLS-stable) with respect to that of GLS in the acute phase (GLS-acute) ≥ 16%; a non-improved GLS indicates the rate of change of the absolute value of GLS-stable with respect to that of GLS-acute < 16%. MACE major cardiovascular events, GLS global longitudinal strain
Discussion
The main results of this study were as follows: First, the rate of change in GLS for patients with MACE was lower than that for those without. Second, after adjusting for confounding variables, the rate of change in GLS, not GLS-stable, was a potent prognostic indicator in patients with HFpEF and acute decompensated HF. Finally, having a non-improved GLS (a rate of change in GLS < 16%) was correlated with the high risk of MACE. Therefore, the changes in GLS could be useful for the long-term risk stratification in patients hospitalized for HFpEF and persistently preserved LVEF.
Buggey et al. showed abnormal GLS values on admission were associated with poor outcomes at 30 days but not by 1 year in patients hospitalized for HFpEF [4]. Several studies have found GLS in the chronic setting is a predictor of HF-related hospitalization and cardiac death among patients with HFpEF [6, 7]. Generally, echocardiographic parameters in patients with HF are not static but are changeable by disease progression or with treatment during follow-up. Therefore, it may be more informative to evaluate the changes in several clinical parameters. A previous study showed that the measurements of serial changes in LVEF provided additional prognostic information in patients with acute decompensated HF [12]. In addition, we previously reported the clinical utility of changes in the left atrial volume index during hospitalization among patients with a first acute myocardial infraction [13]. The present study demonstrated the first evidence of the usefulness of acute-to-stable phase changes in GLS as long-term prognostic indicators in patients hospitalized for HFpEF, especially those with an LVEF ≥ 50% in both the acute and stable phases.
GLS has been reported to be a sensitive marker of early subtle abnormalities of LV myocardial performance, which is helpful for predicting outcomes for various cardiac diseases [14,15,16,17,18]. For 447 patients with HFpEF enrolled in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, Shah et al. found an association between treatment with spironolactone and a trend toward improvement of GLS [7]. Tanaka et al. showed that dapagliflozin significantly improved GLS 6 months after administration in type 2 diabetes mellitus patients with stable HF [19]. A recent study reported that empagliflozin reduced the combined risk of cardiovascular death or hospitalization for heart failure in HFpEF patients [20]. Some studies recommended to use several cardio-protective drugs for HFpEF, such as sodium glucose cotransporter 2 (SGLT2) inhibitors, mineralocorticoid receptor antagonists (MRA) and renin–angiotensin system inhibition with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) or angiotensin receptor-neprilysin inhibitor (ARNI), to decrease the risk of cardiovascular death or hospitalization for HF [21,22,23,24]. Considering our results, patients with a non-improved GLS may be recommended for more aggressive treatment with these drugs.
NT-proBNP level in patients with MACE was significantly higher than that in patients without MACE, and left ventricular end-diastolic pressure (LVEDP) was expected to be high even in the stable phase. Wu et al. reported that LVEDP was positively correlated with GLS [25]. In the MACE group, there may have been less improvement in the longitudinal contractility from acute to stable phase along with GLS. On the other hand, patients with non-MACE have improved the contractility shown as GLS improvement. Reduced LVEDP may have contributed to the improvement of GLS.
Multivariable logistic regression models including systolic blood pressure, E/e′, left ventricular mass index (LVMI), and NT-proBNP in the acute and stable phase revealed that only systolic blood pressure in the acute phase was an independent predictor of improved GLS. As Soufi et al. reported that hypertension is associated with a reduced GLS [26], systolic blood pressure in the acute phase might have affected GLS value. However, there was no correlation between a rate of change in GLS and that in systolic blood pressure from acute to stable phase (data not shown). Therefore, improvement of GLS might be caused not only by decreased blood pressure but also other factors.
Tadic et al. reported that LV hypertrophy is known to be an important morphological change in HFpEF and associated with a reduced GLS [27]. However, on univariate analysis, interventricular septum thickness (IVST), posterior wall thickness (PWT) and LVMI in the acute and stable phase were not associated with MACE. In addition, LVMI in the both acute and stable phase were not correlated with improved GLS. In our study, LV hypertrophy was relatively mild, which may have led to the result that these parameters were not associated with prognosis and improved GLS.
Previous study showed abnormal GLS values on admission were associated with poor outcomes at 30 days. However, they were not predictors of longer outcomes in hospitalized patients with HFpEF [4], which may be consistent with our study. We examined not short-term but long-term outcomes, which may have led to the result that baseline GLS was not a prognostic factor.
Clinical implications
To the best of our knowledge, there have been no prior studies specifically evaluating the association between the changes in GLS values and clinical outcomes in patients hospitalized for HFpEF and persistently preserved LVEF. Our data showed the rate of change in GLS, not GLS in acute and stable phase alone, was significant predictor of MACE, suggesting that serial measurements of GLS is useful to stratify the risk of patients with HFpEF. In addition, patients with low rate of change in GLS had poor prognosis, even if the patients had preserved LVEF. Therefore, patients with a non-improved GLS may be recommended for more careful follow-up and aggressive treatment with agents, such as MRA, SGLT2 inhibitors, ACE inhibitors or ARBs or ARIN, regardless of LVEF. Tanaka proposed that GLS, in conjunction with HF stage classification, was more useful for HF patient management as compared to conventional echocardiographic parameters [14]. A GLS-guided strategy using the rate of change in GLS values may thus have the potential for the better management of patients with HFpEF.
Study limitations
Our study has several limitations. First, it was conducted with retrospective analyses at a single-center, resulting in a relatively small number of study subjects. Second, AF is a common complication in HFpEF, but we excluded patients with AF because they were not suitable for strain analysis due to beat-to-beat variability of ventricular cycle length. Brookes et al. reported that myocardial contractility is constantly changing from beat to beat in atrial fibrillation because of the influence of the force-interval relationships [28]. While joint guidelines published by the American Society of Echocardiography and the European Association of Cardiovascular Imaging suggest a minimum of 5 beats in AF patients for strain analysis [29], it is not very practical and we did not store enough echocardiographic images including 5 or more cardiac cycles. Appropriate strain measurement in AF remains to be a challenge to be solved in the further studies. Third, echocardiographic data in the acute phase may have been affected by treatments received prior to hospitalization. Treatments at discharge were not randomized in the present study, making it difficult to evaluate their effects on outcomes. However, there were no significant differences in medications between patients with and without MACE. Thus, it is reasonable to presume that the medications did not significantly affect our results.
Conclusion
The changes in GLS could be useful to stratify the long-term risk in patients hospitalized for HFpEF and persistently preserved LVEF.
References
Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC (2017) Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 70:2476–2486
Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M (2004) Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J 25:1214–1220
Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H, Investigators JCARE-CARD (2009) Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 73:1893–1900
Buggey J, Alenezi F, Yoon HJ, Phelan M, DeVore AD, Khouri MG, Schulte PJ, Velazquez EJ (2017) Left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: outcomes following an acute heart failure hospitalization. ESC Heart Fail 4:432–439
Park JJ, Park JB, Park JH, Cho GY (2018) Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 71:1947–1957
Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H (2015) Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography 32:71–78
Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD (2015) Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 132:402–414
Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D (1993) Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 88:107–115
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Quantification Writing Group, American Society of Echocardiography’s Guidelines and Standards Committee, European Association of Echocardiography (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18:1440–1463
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Yamamoto M, Seo Y, Ishizu T, Nishi I, Hamada-Harimura Y, Machino-Ohtsuka T, Sato K, Sai S, Nakatsukasa T, Sugano A, Baba M, Obara K, Aonuma K, Ieda M (2019) Different impact of changes in left ventricular ejection fraction between heart failure classifications in patients with acute decompensated heart failure. Circ J 83:584–594
Sakaguchi E, Yamada A, Sugimoto K, Ito Y, Shiino K, Takada K, Iwase M, Ozaki Y (2011) Prognostic value of left atrial volume index in patents with first acute myocardial infarction. Eur J Echocardiogr 12:440–444
Tanaka H (2019) Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management. J Echocardiogr 17:17–24
Gorcsan J 3rd, Tanaka H (2011) Echocardiographic assessment of myocardial strain. J Am Coll Cardiol 58:1401–1413
Stanton T, Leano R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2:356–364
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100:1673–1680
Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S (2010) Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 23:1019–1024
Tanaka H, Soga F, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI (2020) Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol 19:6
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR-Preserved Trial Investigators (2021) Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 385:1451–1461
Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B (2015) Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation 131:34–42
Khan MS, Fonarow GC, Khan H, Greene SJ, Anker SD, Gheorghiade M, Butler J (2017) Renin–angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Fail 4:402–408
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, CHARM Investigators and Committees (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet 362:777–781
Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP, PARAGON-HF Investigators and Committees (2019) Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 381:1609–1620
Wu T, Zheng L, Zhang S, Duan L, Ma J, Zha L, Li L (2022) Left ventricular long-axis ultrasound strain (GLS) is an ideal indicator for patients with anti-hypertension treatment. Clin Exp Hypertens 44:20–25
Soufi Taleb Bendiab N, Meziane-Tani A, Ouabdesselam S, Methia N, Latreche S, Henaoui L, Monsuez JJ, Benkhedda S (2017) Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur J Prev Cardiol 24:1463–1472
Tadic M, Sala C, Carugo S, Mancia G, Grassi G, Cuspidi C (2021) Myocardial strain and left ventricular geometry: a meta-analysis of echocardiographic studies in systemic hypertension. J Hypertens 39:2297–2306
Brookes CI, White PA, Staples M, Oldershaw PJ, Redington AN, Collins PD, Noble MI (1998) Myocardial contractility is not constant during spontaneous atrial fibrillation in patients. Circulation 98:1762–1768
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–271
Acknowledgements
The authors are grateful for the support of the entire staff of the Department of Cardiology, Fujita Health University School of Medicine, Toyoake, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sakaguchi, E., Yamada, A., Naruse, H. et al. Long-term prognostic value of changes in left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction. Heart Vessels 38, 645–652 (2023). https://doi.org/10.1007/s00380-022-02211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02211-y