Abstract
The study was designed to assess the accuracy of contrast-enhanced balanced steady-state free precession (cine-SSFP) CMR imaging sequences to exhibit myocardial hyperemia in acute myocarditis, which has for a long time been investigated in some centers using early gadolinium enhancement (EGE) sequence. Contrast-enhanced cine-SSFP (CESSFP) sequences were compared to precontrast cine-SSFP sequences to calculate the early cine-contrast enhancement in 36 consecutive patients with acute myocarditis and 36 controls matched for age and gender. Four-chamber views images were obtained in each subject before and after gadolinium injection. Absolute and relative left ventricular myocardial enhancement of the overall myocardium, then separately of the lateral wall and interventricular septum was analyzed in telediastole. Myocarditis patients displayed higher cine-SSFP absolute enhancement than controls (overall left ventricular myocardium 2.38 ± 0.33 vs 1.84 ± 0.31; lateral wall 2.45 ± 0.35 vs 1.83 ± 0.32; and septum 2.26 ± 0.29 vs 1.82 ± 0.29, p < 0.0001 for all). Less significant differences were observed for the relative enhancement (p < 0.05 for all). Using ROC curves, the optimal threshold value of absolute enhancement to diagnose acute myocarditis was 2.05 (sensitivity: 86%; specificity: 81%). Given the simplicity of use, contrast-enhanced cine-SSFP sequences should be used as an additional diagnostic tool to detect hyperemia in acute myocarditis patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac MR imaging (CMR) has the capabilities to identify cardiac disease by comparing global and regional contractile function, perfusion conditions (with early first-pass perfusion study using contrast agents) and/or myocardial scar or inflammation estimated by delayed retention of a contrast agent (late contrast–enhancement after gadolinium chelate injection) [1,2,3,4,5]. CMR has hence emerged as a crucial non-invasive tool for the diagnosis and monitoring of myocarditis. In 2009, the Lake Louise Consensus Group recommended a standard protocol that would best identify myocardial involvement using CMR [6]. Diagnostic targets for the three recommended CMR criteria were edema, hyperemia, capillary leak, and necrosis. Two out of three “Lake Louise Criteria” allowed for a correct diagnosis of acute myocarditis in about 80% of cases [6, 7]. The 2009 Lake Louise Criteria (LLC) required time-consuming specific acquisitions, including T2-weighted sequences for edema assessment, T1-weighted pre- and early post-Gadolinium enhancement (EGE) sequences for inflammation/hyperaemia/capillary leak detection, and late Gadolinium enhancement imaging (LGE) for myocyte injury/necrosis and subsequent fibrosis evaluation [6]. In 2018, new criteria were proposed.
The main problem was that the early gadolinium enhancement T1-weighted SE sequence recommended for 2009 LLC hyperemia assessment was imperatively performed with the body coil before and 4 min. after gadolinium chelate administration for accurate enhancement measurements [8]. Hyperemia detection by this old technique was hampered by the acquisition length and inconsistent image quality. Moreover, the quantitative analysis recommending an enhancement ratio cut-off > 2.5” in patients with myocarditis did not provide information about regional distribution of myocardial involvement, and was the least accurate among other CMR parameters [5, 9]. Cine magnetic resonance imaging (cine MR) using cineSSFP sequences is considered the standard of reference for measurement of ventricular volume and various parameters of global ventricular function, such as ejection fraction and ventricular mass [10,11,12,13]; cineSSFP also has intrinsic properties of T2/T1 contrast that may be used to study contrast enhancement [14,15,16,17,18].
Even if the 2009 LLC have been updated in 2018, with a recent statement that includes T1- and T2-weighted images and does no longer suggest using the early gadolinium enhancement [19], assessment of hyperemia may have a residual role in myocarditis diagnosis. We aimed to investigate whether post-contrast cine-SSFP acquisitions provided useful qualitative and quantitative data upon regional hyperemia compared to precontrast cine MR images, in a cohort of patients with acute myocarditis.
Materials and methods
Patient selection
Between March 2018 and May 2019, all patients referred for CMR at our institution had both pre and post contrast 4-chamber cine MR sequence included in their comprehensive CMR examination. The study was approved by the local ethics committee and complied with the Declaration of Helsinki. All patients enrolled in this study were required to understand and give their consent for participation.
Diagnosis of acute myocarditis was based on the combination of first, a raised high-sensitivity cardiac troponin T (hs-cTnT) and at least 2 of the following criteria: continuous chest pain > 10 min., normal coronary imaging (based on coronary computed tomography or invasive coronary angiography) or no cardiovascular factors, and second, of the modified CMR-LLC [19] including presence of 2 of the 3 following criteria: depiction of edema on T2-weighted images, increased T1 and/or T2 mapping values, and myocardial injury (subepicardial or midwall lesions, sparing of the subendocardium) on late Gadolinium enhancement (LGE). Basically, this new recommendation is based on the association of T2- and T1-weighted anomalies, T2 representing edema while abnormal T1 unveils myocyte injury. In our center, we used only high-sensitivity cardiac troponin I (hs-cTnI) (Abbott Diagnostics, ARCHITECT STAT) with a negative hs-cTnI defined by a value < 5 ng/L, and the 99th percentile was 52 ng/l.
Thirty-nine consecutive patients undergoing MR imaging for an ongoing acute coronary syndrome highly suggestive of acute myocarditis (16 men, aged 39 ± 10.5 years) were selected. No patient was recruited with a cardiomyopathy-like presentation. Three of these myocarditis patients initially eligible for study were excluded because incomplete or inconsistent CMR examination (i.e., arrhythmia or breathing and moving artifacts leading to incoherent pre- and post-contrast image colocalization). Thus, the 36 remaining myocarditis patients were compared to 36 controls matched for age and gender. Controls were recruited as symptomatic patients (chest pain or dyspnea without evidence of myocarditis or other cardiovascular diseases after all investigations were performed: negative hs-cTnI (< 5 ng/L), ECG without repolarization abnormalities in sinus rhythm, and no CMR abnormalities.
Study protocol
All patients were examined in supine position, and breath-hold was obtained at tidal inspiration.
MR examinations were performed on a 1.5 T system (Siemens Aera, Erlangen, Germany) with a 32 channel dedicated phased-array cardiac coil.
The comprehensive imaging protocol included (a) Gradient echo scout localization imaging of the chest to locate the long axis, then the short axis and four chamber views. (b) short axis and 4-chamber breath-hold precontrast cineSSFP acquisitions, (c) T1 and T2 mapping in short axis (basal, midventricular and apical locations), (d) first-pass perfusion short axis, long axis 2- and 4-chamber contrast-enhanced gradient-echo T1-weighted acquisitions (e) long axis 2- and 4-chamber breath-hold contrast-enhanced (CE) cineSSFP acquisitions, and (f) contrast-enhanced gradient-echo T1-weighted late gadolinium enhancement (LGE) acquisitions with an inversion pulse to null the signal of normal myocardium and PSIR images in the three cardiac planes. All the acquisitions were cardiac gated. Precontrast and CE cineSSFP acquisitions, 3 min after injection of 0.2 mmol/kg of Gadoterate dimeglumine (Dotarem®; Guerbet, Aulnay-sous-Bois, France) used the same following parameters: field of view (FOV), 320–340 mm according to the individual size; repetition time (TR), 3.4 ms; echo time (TE), 1.3 ms; flip angle (FA), 50 degrees; matrix size, 256 × 224 with interpolation reconstruction of 512 × 512; slice thickness 8 mm with an interslice gap of 10%; temporal resolution, around 42 ms with 25 phases per section, according to the heart rate.; reconstruction of in plane spatial resolution, 1.4 × 1.3 mm. Three contiguous slices were acquired in long axis views and 5 to 7 slices in short axis views.
The standardized localizer protocol and the use of papillary muscles as landmarks helped to ensure the reproducibility of the slice location between acquisitions.
Data interpretation
CMR assessment of inflammation/hyperemia
Qualitative and quantitative interpretation of each MR dataset was performed in two successive sessions by 2 MR readers experienced in cardiac imaging separately, then in consensus in case of discordant measures (TP, JPL). The two readers were blinded to clinical data and to the results of the other reading sessions. For this purpose, studies were presented anonymously.
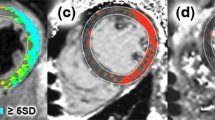
Using commercially available software (Syngovia, Siemens, Erlangen Germany), myocardial signal intensities were measured slice by slice on 4-chamber views by manually tracing two irregular regions of interest (ROIs) on the whole septum and lateral wall (Fig. 1). The ROIs defined on CE-SSFP images were copied and transferred 1:1 to precontrast images to perfectly colocate identical myocardial regions for calculation of the absolute and relative enhancement.
Skeletal muscle signal was measured as reference by tracing a 1 cm2 round-shaped ROI in muscular tissue of the latissimus dorsi or erector spinae muscle included in the field of-view.
Global then regional lateral wall and septal absolute and relative enhancement were computed from SSFP frames in end-telediastole before and after Gd chelate injection.
LGE was qualitatively assessed as present and compatible with myocarditis, or absent.
The signal intensity (SI) measured in the whole left ventricular myocardium and in skeletal muscle in the same slice (latissimus dorsi) then in the septum and lateral wall separately on each SSFP measurement allowed to calculate the relative signal enhancement as follows:
Absolute enhancement = (SImyocardium post- SImyocardium pre)/SImyocardium pre.
The relative enhancement (or enhancement ratio) was determined by normalizing myocardial enhancement to skeletal muscle enhancement using the same formulas for myocardium as for muscle.
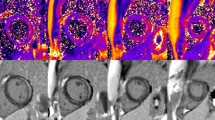
T1 and T2 mapping
Parametric T1 and T2 values were measured in the septum and lateral wall using free hand ROIs. T1 and T2 relaxation times were extracted from corresponding T1 and T2 maps, analyzed using a segmental approach (Fig. 2).
Pre and post-contrast 4-chamber end-diastolic cineSSFP images (a, b), short axis T1 (c) and T2 (d) map images, 4-chamber (e) and short axis (f) LGE images, and short axis PSIR (g) image of a patient with acute myocarditis. Subepicardial lateral wall enhancement is obvious on CE cineSSFP images and LGE images (arrowheads). T2 mapping shows lateral wall edema whereas a slight T1 increase on T1 mapping is present in the same location
Statistics
All results are given as mean and standard deviations (SD) or as median (IQR) when at least some data were not normally distributed. Depending on whether the distribution was different from normality, the significance of differences was analyzed using Student’s unpaired t-test, Wilcoxon test or Mann–Whitney rank sum test, where appropriate. As LGE was only recorded as a binary variable (positive or negative), Fisher’s exact test was used for comparison. Two-tailed p values < 0.05 were considered statistically significant. Linear regression analysis was used to compare absolute myocardial enhancement with T1 and T2 values.
Receiver operating curve (ROC) analysis was used to assess the diagnostic performance of contrast-enhanced cineSSFP. Sensitivity and specificity were computed and the cut-off values derived from receiver operating characteristic (ROC) analysis for the absolute and relative enhancement values.
The delay between contrast injection and post-contrast cineSSFP acquisitions was compared between patients and controls.
All statistics were calculated using commercially available software (GraphPad Prism version 7, Graphpad Software Inc, San Diego, USA).
Observer assessment
For the assessment of inter- and intra-observer variability, absolute and relative enhancement were calculated in 20 randomly selected examinations (in 10 myocarditis and 10 controls) at two time points at least one week apart by the first reader, and once by the second reader blinded to the first reader’s results. For the evaluation of intra-observer and inter-observer variability, the intra-class correlation coefficient was used.
Results
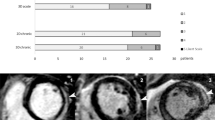
Clinical features at presentation included a typical acute chest pain of < 12 h duration in all myocarditis patients (Table 1). A recent viral illness involving the ENT and/or upper respiratory tract was identified in 20 patients (56%). Fever was present in 14 patients (39%). and pericardial symptoms were noted in 10 (28%). None of the myocarditis patients were unstable on admission requiring ICU transfer. Among 28 patients with altered ECGs, ischemic ECG abnormalities (ST segment depression) were present in nine patients (25%); typical ECG signs of pericarditis were present in 6 (17%). As seen above, patients with arrhythmia were excluded. For comparison, clinical presentation of controls is summarized in Table 1.
Time delay between contrast Gd injection and the first post-contrast cineSSFP slice acquisition was not significantly different between myocarditis patients and controls (3.51 ± 1.63 mn vs 3.12 ± 1.08 mn, p = 0.76).
Overall myocardial enhancement
In myocarditis patients, the ceSSFP myocardial absolute enhancement ranged from 1.87 to 3.5, respectively, for the whole myocardium; the corresponding mean values were 2.38 ± 0.33, and median values were 2.34 (IQR 2.13–2.67). The absolute enhancement of the skeletal muscle was 1.48 ± 0.28. In controls, the corresponding absolute enhancement was homogeneous and ranged from 1.31 to 2.98 for the whole myocardium; the corresponding mean values were 1.84 ± 0.31, and median values 1.77 (IQR 1.62–1.98) (Fig. 3). The absolute enhancement of the skeletal muscle was 1.41 ± 0.26. The relative enhancement values were also greater in patients than in controls (Table 2). Overall, the difference (Fig. 3) was significant (2.9 ± 1.86 in myocarditis patients vs 2.00 ± 1.78 in controls, p < 0.05).
Regional myocardial enhancement
Enhancement of the lateral wall and septum was significantly higher in myocarditis patients compared to controls (Fig. 4). Additionally, there was a statistical difference (Table 2) of absolute and relative enhancement between the lateral wall and the septum (p comprised between 0.001 and 0.03) in myocarditis patients but not in controls (Fig. 4).
Enhancement accuracy
From the ROC analysis as illustrated in Fig. 5, the area under the curve (AUC) obtained for cine-SSFP enhancement was 0.90 ± 0.03 (95% confidence interval, 0.83–0.96). The sensitivity and specificity were 86% and 81% respectively, using a cut-off absolute enhancement threshold of 2.05.
The inter- and intra-observer variability intra-class correlation coefficient (ICC) values for absolute enhancement were 0.95 and 0.96. The inter- and intra-observer variability ICC values for absolute enhancement were 0.94 and 0.96.
Relationship with T1 and T2 mapping
Myocarditis patients displayed increased global and regional T2 values compared to controls (global T2 time: 53.6 ± 5.4 ms vs. 48.6 ± 2.7 ms, p < 0.001; lateral wall T2 time 56.2 ± 4.9 ms vs. 48.4 ± 2.5 ms, p = 0.0001). In myocarditis, T1 values were also significantly increased overall and in the lateral wall (1049 ± 81 ms vs 1009 ± 45 ms, p = 0.04; and 1071 ± 81 ms vs 1008 ± 51 ms, p = 0.01). Differences in T2 and T1 relaxation times in the septum did not reach significance (Table 3).
There was no correlation (Fig. 3) between T1, T2 values and the level of absolute or relative enhancement (r = − 0.0362, p = 0.83 and r = − 0.1618, p = 0.35).
Discussion
This study shows that myocarditis patients displayed higher cine-SSFP absolute and relative enhancement than controls, in particular in the lateral LV wall. Moreover, absolute enhancement by itself was as accurate as relative enhancement, which simplifies calculations since there is no need to record muscle signal.
The major interest of performing CE-cineSSFP in patients with acute chest pain is the reduction of the CMR examination time whatever the underlying cause of disease, resulting in an expected better participation from patients and an optimal throughput. From the present study intended to confirm the diagnostic utility of this sequence, adding precontrast to contrast-enhanced 4-chamber SSFP images consequently represents a major advantage when compared to the literature on EGE to study the early enhancement ratio as recommended in 2009 Lake Louise criteria where this sequence was dedicated exclusively to acute myocarditis [17,18,19,20]. Moreover, the capability to differentiate signal intensity from areas close to myocarditis hyperemia and those of remote normal myocardium represents another advantage of the technique.
The time delay for achieving the best contrast between the injured and normal myocardium has been suggested to be 5 mn [21] using the sequence of Simonetti et al. [22], to catch the inflammation hyperemia [20, 21].
CineSSFP sequences have been primarily used to achieve excellent contrast between myocardium and cardiac chambers, with a high temporal resolution [10, 11]. The signal on cineSSFP images is closely dependent on the T2-to-T1 ratio, which is higher in segments mixing edema and injured cells than in normal myocardium, despite remaining lower than in circulating blood. The cineSSFP sequence can be used for wall motion studies as well as for identification of segments in which Gd-chelate clearance is altered. Early hyperenhancement of abnormal myocardial areas on cineSSFP is thus expected to have significantly higher T1 signal than the remote normal myocardium. These properties of CE-cineSSFP sequences have been used in patients with myocardial infarction, with results similar to those obtained with delayed-enhancement sequences [1, 14, 15], and in patients with acute myocarditis [9, 17, 18]. Hence, when 4-chamber cine sequences are obtained early after first pass perfusion studies, they can act in a similar way as early gadolinium enhancement sequence proposed by Friedrich et al. 20 years ago [8] and included in the 2009 LLC, and should be an adjunct to the 2018 LLC in difficult cases. A greater sensitivity and specificity is achieved at the expense of a reduced CMR examination duration. Our study also showed that separate ROI measurements in the septum and lateral wall better discriminate myocarditis patients from controls.
It has been reported that hyperemia and/or edema with EGE are more obvious in areas of LGE [18]. Any tissue inflammation with preserved vasculature is associated with an increased regional blood flow and volume. In acute myocarditis, this has been used as a diagnostic criterion using contrast-enhanced CMR images obtained early after administration of gadolinium with its initial vascular and brief interstitial steady state [16, 17]. In these “early enhancement” images, inflamed regions appear with higher signal intensity. Because cardiac output, renal clearance, artifacts, and internal signal post-processing may alter absolute signal intensity, published studies successfully used values normalized to skeletal muscle [16, 18]. In the case of suspected skeletal muscle involvement, however, the contrast agent-induced relative signal intensity increase was found to be more sensitive without normalization [17]. That is the reason why calculations were made with and without skeletal normalization.
Despite displaying less important differences between myocarditis patients and controls, abnormal early septum enhancement was present simultaneously with lateral wall enhancement; these data had been observed previously, suggesting a more extensive myocardial enhancement than shown by LGE [23].
Assessment of the absolute and relative enhancement can only act as an adjunct to novel techniques, notably T1 and T2 mapping, including extracellular volume quantification, which have emerged showing significant improvement in the characterization of the myocardium. These parametric CMR approaches quantifying native T1 and T2 relaxation time and myocardial texture have increased CMR-based diagnostic accuracy for acute myocarditis [23,24,25,26]. In those patients, these advancements appear to overcome some of the limitations of the 2009 Lake Louise Criteria. [27, 28].
Even if native T1 and T2 mapping is increasingly performed in the setting of acute myocarditis, early post-contrast cine-SSFP imaging remains routinely used [17,18,19,20].
Limitations
Post contrast cineSSFP images were not compared and validated vs the standard method for EGE imaging in the present study, so direct comparison of the new technique to the old one is not possible.
The colocalization of T1 and T2 mapping images on one hand, and of cineSSFP images on the other hand was not strictly in the same areas, which can be an explanation to the absence of correlation between relaxation time and enhancement values.
As in many previous studies, histological proof of myocarditis was not available. Indeed, to validate diagnostic criteria for acute myocarditis, the diagnosis of myocarditis should rely on a gold standard such as biopsy. The criteria that are given in the present methodology are derived from CMR only, and cannot differentiate patients with MINOCA or myocarditis.
Only post-contrast 4-chamber views were obtained; this is a pitfall given that myocarditis affects notably the posterior lateral wall of the LV. Depending on the section orientation chosen for SI measurements, some myocardial segments can escape image analysis, which is deleterious in case of involved segments (23). However, this acquisition plane was chosen as a trade-off between functional cardiac assessment needs and the comparison with the strict axial plane used by Friedrich et al. to calculate EGE (8). No attempt was made to calculate the incremental value of CE SSFP enhancement to the diagnostic yield of a comprehensive CMR examination. Another limitation of our study is the relatively small sample size that potentially could have resulted in data heterogeneity, even if the study population was large enough to obtain statistically significant results. In particular, the threshold for absolute enhancement should be validated in an external and large cohort.
At last, it is unclear whether performing the stack of short-axis cine images to measure LVEF after contrast administration as in many institutions would have been altered these results.
In summary, contrast-enhanced cineSSFP sequence offers the advantage to be available in any clinical indication where cardiac function needs to be studied, and to reduce the overall examination time because it can be performed between the first-pass and delayed enhancement sequences. Hence, this sequence should be used alternatively or in conjunction to noncontrast cineMR sequences for routine MR assessment of myocarditis and could act as a possible adjunct within the armamentarium of a comprehensive myocarditis CMR examination.
Abbreviations
- CE:
-
Contrast-enhanced
- CMR:
-
Cardiac magnetic resonance
- LV:
-
Left ventricular
- SSFP:
-
Steady-state free precession
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
References
Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK (2007) The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur Heart J 28:1242–1249
Gerber BL, Raman SV, Nayak K, Epstein FH, Ferreira P, Axel L, Kraitchman DL (2008) Myocardial first-pass perfusion cardiovascular magnetic resonance: history, theory, and current state of the art. J Cardiovasc Magn Reson 10:18
Masci PG, Dymarkowski S, Bogaert J (2008) The role of cardiovascular magnetic resonance in the diagnosis and management of cardiomyopathies. J Cardiovasc Med (Hagerstown) 9:435–449
Karamitsos TD, Francis JM, Neubauer S (2011) The current and emerging role of cardiovascular magnetic resonance in the diagnosis of nonischemic cardiomyopathies. Prog Cardiovasc Dis 54:253–265
Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for cardiovascular magnetic resonance board of trustees task force on standardized protocols (2013) Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 15:91
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International consensus group on cardiovascular magnetic resonance in myocarditis (2009) Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53:1475–1487
Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, Fuernau G, de Waha S, Sareban M, Luecke C, Klingel K, Kandolf R, Schuler G, Gutberlet M, Thiele H (2012) Diagnostic performance of CMR imaging compared with EMB in patients with suspected myocarditis. JACC Cardiovasc Imaging 5:513–524
Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R (1998) Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 97:1802–1809
Chu GC, Flewitt JA, Mikami Y, Vermes E, Friedrich MG (2013) Assessment of acute myocarditis by cardiovascular MR: diagnostic performance of shortened protocols. Int J Cardiovasc Imaging 29:1077–1083
Barkhausen J, Ruehm SG, Goyen M, Buck T, Laub G, Debatin JF (2001) MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low angle shot cine MR imaging—feasibility study. Radiology 219:264–269
Carr JC, Simonetti O, Bundy J, Li D, Pereles S, Finn JP (2001) Cine MR angiography of the heart with segmented true fast imaging with steady state precession. Radiology 219:828–834
Thiele H, Nagel E, Paetsch I, Schnackenburg B, Bornstedt A, Kouwenhoven M, Wahl A, Schuler G, Fleck E (2001) Functional cardiac MR imaging with steady-state free precession (SSFP) significantly improves endocardial border delineation without contrast agents. J Magn Reson Imaging 14:362–367
Scheffler K, Lehnhardt S (2003) Principles and applications of balanced SSFP techniques. Eur Radiol 13:2409–2418
Setser RM, Kim JK, Chung YC, Chen K, Stillman AE, Loeffler R, Simonetti OP, Weaver JA, Lieber ML, White RD (2006) Cine delayed-enhancement MR imaging of the heart: initial experience. Radiology 239:856–862
Kim KA, Seo JB, Do KH, Heo JN, Lee YK, Song JW, Lee JS, Song KS, Lim TH (2006) Differentiation of recently infarcted myocardium from chronic myocardial scar: the value of contrast-enhanced SSFP-based cine MR imaging. Korean J Radiol 7:14–19
Laissy JP, Hyafil F, Huart V, Chillon S, Schouman-Claeys E, Faraggi M (2005) Value of contrast-enhanced, balanced cine-MR sequences in the assessment of apparent infarct size after acute myocardial infarction: a prospective comparison with delayed-enhancement sequences. J Magn Res Imaging 22:765–771
Deux JF, Maatouk M, Lim P, Vignaud A, Mayer J, Gueret P, Rahmouni A (2011) Acute myocarditis: diagnostic value of contrast-enhanced cine steady-state free precession MRI sequences. AJR Am J Roentgenol 197:1081–1087
Perfetti M, Malatesta G, Alvarez I, Liga R, Barison A, Todiere G, Eletto N, De Caterina R, Lombardi M, Aquaro GD (2014) A fast and effective method toassess myocardial hyperemia in acute myocarditis by magnetic resonance. Int J Cardiovasc Imaging 30:629–637
Ferreira VM, Schulz-Menger J, Holmvang KCM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 72:3158–3176
Zarka S, Bouleti C, Arangalage D, Chopra H, Chillon S, Henry-Feugeas MC, Abtan J, Juliard JM, Iung B, Vahanian A, Laissy JP, Ou P (2016) Usefulness of subepicardial hyperemia on contrast-enhanced first-pass magnetic resonance perfusion imaging for diagnosis of acute myocarditis. Am J Cardiol 118:440–445
Jacquier A, Prost C, Amabile N, Giorgi R, Flavian A, Gaubert JY, Varoquaux A, Paganelli F, Bartoli JM, Moulin G (2011) Gadolinium chelate kinetics in cardiac MR imaging of myocarditis: comparison to acute myocardial infarction and impact on late gadolinium enhancement. Invest Radiol 46:705–710
Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM (2001) An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218:215–223
Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, Fimmers R, Stehning C, Homsi R, Schwab JO, Schild H, Naehle CP (2014) Acute myocarditis: multiparametric cardiac MR imaging. Radiology 273:383–392
Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K (2015) Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 8:e003073–e003073
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos T, Francis J, Ntusi N, Holloway C, Choudhury R, Kardos A, Robson M, Friedrich M, Neubauer S (2013) T1 Mapping for the diagnosis of acute myocarditis using CMR: Comparison to T2-Weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging 6:1048–1058
Baessler B, Luecke C, Lurz J, Klingel K, Das A, von Roeder M, de Waha-Thiele S, Besler C, Rommel KP, Maintz D, Gutberlet M, Thiele H, Lurz P (2019) Cardiac MRI and texture analysis of myocardial T1 and T2 maps in myocarditis with acute versus chronic symptoms of heart failure. Radiology 292:608–617
Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, Schmeel FC, Sprinkart AM, Thomas DK (2019) Comparison of original and 2018 lake louise criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol Cardiothorac Imaging 1(3):e190010
Chetrit M, Friedrich MG (2018) The unique role of cardiovascular magnetic resonance imaging in acute myocarditis. F1000Res 7:1153
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laissy, JP., Pezel, T., Herbin, C. et al. Contrast-enhanced cine MR sequences in the assessment of myocardial hyperemia in acute myocarditis: can they help? A feasibility study. Heart Vessels 38, 662–670 (2023). https://doi.org/10.1007/s00380-022-02207-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-022-02207-8