Abstract
Calcific aortic valve disease (CAVD) is the most common heart valve disease requiring intervention. Most research on CAVD has focused on inflammation, ossification, and cellular phenotype transformation. To gain a broader picture into the wide range of cellular and molecular mechanisms involved in this disease, we compared the total protein profiles between calcified and non-calcified areas from 5 human valves resected during surgery. The 1413 positively identified proteins were filtered down to 248 proteins present in both calcified and non-calcified segments of at least 3 of the 5 valves, which were then analyzed using Ingenuity Pathway Analysis. Concurrently, the top 40 differentially abundant proteins were grouped according to their biological functions and shown in interactive networks. Finally, the abundance of selected osteogenic proteins (osteopontin, osteonectin, osteocalcin, osteoprotegerin, and RANK) was quantified using ELISA and/or immunohistochemistry. The top pathways identified were complement system, acute phase response signaling, metabolism, LXR/RXR and FXR/RXR activation, actin cytoskeleton, mineral binding, nucleic acid interaction, structural extracellular matrix (ECM), and angiogenesis. There was a greater abundance of osteopontin, osteonectin, osteocalcin, osteoprotegerin, and RANK in the calcified regions than the non-calcified ones. The osteogenic proteins also formed key connections between the biological signaling pathways in the network model. In conclusion, this proteomic analysis demonstrated the involvement of multiple signaling pathways in CAVD. The interconnectedness of these pathways provides new insights for the treatment of this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcific aortic valve disease (CAVD) is the most common valvular heart disease requiring intervention and results in aortic stenosis (AS). Currently, there is no effective therapy other than surgical or transcatheter valve replacement. CAVD is now recognized to be an actively regulated process [1, 2], rather than a passive degenerative disease in which calcium phosphate accumulates in the leaflet tissue to form nodules, as it was once described. Inflammation, ossification, and cellular phenotypic transformation have all been examined to discern their roles in the disease process [3, 4]. However, many questions remain regarding the pathobiological mechanisms of CAVD, which limits the range of strategies for treatment.
Although there have been numerous investigations of selected remodeling and signaling pathways in CAVD, far fewer comprehensive studies of the biology and pathology of this condition exist. In one such study, Martin-Rojas et al. compared the proteome of diseased vs. normal patients, and identified 35 upregulated and 8 downregulated proteins involved in fibrosis, homeostasis, and coagulation [5]. Alterations in the extracellular matrix (proteoglycans, glycosaminoglycans, and collagen especially) have also been reported in human CAVD [6,7,8,9]. An elegant recent work identified differential proteins from the non-diseased, fibrotic, and calcific areas of the valve tissues, representing the stages of CAVD manifestations [10], but other than these reports there has been only limited investigation of CAVD proteomics.
Evidence is growing that the aortic valve (AV) ECM may contribute to disease processes through cell–matrix interactions [11,12,13,14]. VICs receive stimuli from their immediate surroundings and respond to external stimuli by altering their phenotype and their expression of diverse proteins. Thus, a proteomic analysis will reveal how these stimuli drive changes in proteins responsible for cellular mechanisms as well as those with functional roles within the ECM. This analysis can also lead to insights about the contextual relationships among the diverse families of proteins, as well as their functions, and how those change in the setting of disease. Previously the osteogenic proteins osteopontin (OSTP), osteonectin (SPARC), and osteocalcin (OSTCN) were identified and their distribution pattern mapped within calcified carotid tissues [15]. These proteins are heavily involved in cell–matrix interactions in mineralized tissues, and, therefore, are also relevant to CAVD. Therefore, this study is aimed at identifying the proteins most relevant to the biological processes, cell phenotypes, and ECM components involved in the calcification process, and thus improving understanding of the cellular and molecular mechanisms of CAVD. Ultimately, the delineated biological pathways may provide insights for future diagnosis and therapy of CAVD.
Materials and methods
Protein extraction and quantification
Aortic valves were collected from patients undergoing valve replacement surgeries at the Houston Methodist Hospital. Research use of these surgically-resected tissues was approved by the Institutional Review Boards at Houston Methodist Hospital and Rice University and was in accordance with the ethical standards of the IRBs and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all subjects prior to inclusion in the study.
The valves were immersed in ice-cold PBS/glycerol (50:50), transported to the lab, and photographed. Valves were visually assessed and scored to semi-quantitatively evaluate the degree of calcification (scores 0–5; 5 being severe with calcific nodules found in the majority of valve tissue area) and fibrosis (scores 0–5). Valves selected for further evaluation fulfilled the following criteria: (1) moderate calcification (scores of 2 or 3); (2) having three intact leaflets to exclude bicuspid valves; and (3) having comparable non-calcified and calcific areas when viewed from the fibrosa side (Fig. 1). The valve tissue was carefully dissected into overtly non-calcified and overtly-calcified areas (bulging calcified nodules) under a stereo dissecting microscope with the help of a teasing needle.
The valve tissue was rinsed in PBS 3X 20 min on a shaker at 4 °C to remove the glycerol, then dissected into non-calcified (NoCa) and calcified (Ca) areas and weighed. The tissue was homogenized on ice with an upright homogenizer (Brinkmann Polytron) in a 5X volume of T-PER protein extraction reagent (Thermo Scientific) with protease inhibitor (cOmplete™, Roche). The homogenate was centrifuged at 10,000×g for 5 min. The supernatant was collected and stored at − 80 °C until use. Protein concentration was determined using the colorimetric DC Protein Assay (Bio-Rad) against bovine serum albumin standards per the manufacturer’s instructions.
Proteomic sample preparation and LC–MS/MS
The five patient valves selected for proteomic analysis were from four males and one female, all Caucasians, with an average age of 75.0 ± 8.3 years. Protein samples (100 μg) from non-calcified and calcified areas of each valve were separated on 10% SDS-PAGE gels and stained with Coomassie blue according to standard procedures. The gel lanes were precisely excised from the gel slabs and cut into top and bottom halves. The gel band samples were subjected to in-gel digestion as previously described [16].
An aliquot of the tryptic digest (in 2% acetonitrile/0.1% formic acid in water) was analyzed by LC–MS/MS on an LTQ-Orbitrap-XL mass spectrometer (Thermo Fisher) interfaced with an Eksigent Nano-LC-Ultra- 2D plus CHiPLC Nanoflex system (AB SCIEX). The gradient conditions (A: 0.1% formic acid in water; B: 0.1% formic acid in acetonitrile) consisted of 3%–8% B for 5 min, 8%-33% B for 120 min, 33%–90% B for 10 min, 90% B held for 10 min, then 90% -3% B for 5 min for a total run time of 150 min. The LTQ Orbitrap was operated in parallel mode with measurement of the full mass scan at 100,000 resolutions in the Orbitrap, concurrent with the acquisition of the five most intense data-dependent MS/MS scans in the ion trap. In each cycle, MS1 was acquired at target value 1E6, with the MS2 scan at 3E4. The spray voltage was 1.35 kV, with settings enabled for charge state screening and rejection of singly charged ions. For MS2, the settings were ion selection threshold of 8000, 35% normalized collision energy, activation Q of 0.25 and dynamic exclusion for 90 s. Each sample was analyzed in duplicate.
Proteomic data analysis
The raw data files from the MS analysis were processed to generate a Mascot Generic Format using Mascot Distiller (Matrix Science) and then searched against the IPI_human 3.87 protein database (containing 91,464 protein sequences) using Mascot v2.3.02. The spectra were also searched against a decoy database using a target false discovery rate (FDR) of 1% for strict and 5% for relaxed conditions. For trypsin proteolysis, up to two missed cleavages were allowed. MS tolerance was set at 10 ppm; MS/MS tolerance was set at 0.8 Da. Carbamidomethylation on cysteine residues was used as fixed modification, whereas serine, threonine, tyrosine phosphorylation, methionine oxidation, and N-terminal acetylation of protein were set as variable modifications. Proteins identified by a single peptide with Mascot scores lower than 25 were eliminated. The raw data files were also analyzed using Proteome Discoverer 1.3 (Thermo Fisher) to search the spectra against targeted osteogenic proteins of interest (FASTA data files). To account for measurement variations between samples, the Exponentially Modified Protein Abundance Index (emPAI = 10Nobserved/Nobservable-1) values of all proteins were calculated then normalized by dividing by the emPAI value of GAPDH.
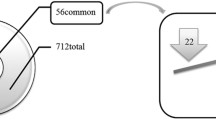
The above process positively identified 1413 unique proteins within the 10 valve samples. A subgroup of 248 proteins common to both the Ca and NoCa regions of either all 5 valves (142), 4/5 valves (49), or 3/5 valves (57) was compiled. For each protein, the Ca/NoCa ratios for each valve were calculated and examined using descriptive statistics. Any protein with a coefficient of variation (CV) > 100% was removed as an outlier, leaving 198 proteins remaining in the filtered data set. The average of the Ca/NoCa ratios were calculated and uploaded as a single value for each protein into Ingenuity Pathway Assist (IPA) v. 7-16-18 (Qiagen). Data were analyzed using standard IPA techniques.
In a parallel analysis, we used the paired Wilcoxon rank test to identify proteins that were differentially abundant between paired calcific and non-calcific tissue samples. Out of 1413 proteins, the top forty ranked proteins were grouped into eight categories according to their known biological functionalities. The differing amounts present of these forty proteins between paired samples were visualized using CIRCOS software [17].
To gain insight into the relationships between these 40 MS-identified proteins and 5 osteogenic proteins of interest in CAVD, we extracted the protein–protein interaction information of these 45 proteins from the String database to build a calcification-specific network [18]. A first-level network expansion was performed to incorporate linker proteins known to associate with the key target proteins. The results revealed one large network, three islands of smaller networks, and lone unconnected proteins. After omitting the lone proteins, the resulting protein network, visualized using Cytoscape [19], included 24 MS-identified proteins, 5 osteogenic proteins, and 42 linker proteins.
Validation of osteogenic protein levels
Enzyme-linked immunosorbent assay (ELISA) kits were used to confirm the levels of selected osteogenic proteins in the valves. This analysis was performed on protein from the 5 valves from the MS analysis and an additional 5 patient valves (4 males, 1 female, all Caucasians); the average age of the entire group was 72.0 ± 8.8 years. Osteopontin (Quantikine, R&D Systems) and osteonectin/SPARC (Adipo Bioscience) were measured from the protein extracts of the calcified and non-calcified regions of valve tissues per manufacturers’ recommendations and compared using a Wilcoxon matched-pairs test (GraphPad Prism).
Immunohistochemistry of osteogenic proteins
The presence of several osteogenic proteins was assessed in normal aortic valve tissues from three cadaver tissues (Cooperative Human Tissue Network) and compared with calcified leaflet tissues from three patients who underwent valve replacement surgeries. Paraformaldehyde-fixed tissues were paraffin-embedded and sectioned (5 μm). Following an antigen retrieval step (0.01 M citric acid), sections were processed through standard immunohistochemical procedures to detect osteopontin (MAB1433, R&D Systems), osteonectin/SPARC (MAB941, R&D Systems), osteocalcin (MAB1419, R&D Systems), osteoprotegerin (MA5-15,960, Thermo Scientific), and RANK/CD265 (9A725, Thermo Scientific). Stained samples were scored for their degree of staining as follows: 0 = absent, 1 = weak (< 33.3%), 2 = moderate (33.3–66.7%), and 3 = strong (> 66.7%) area. The scores were assigned by two trained observers independently. Intra-observer variability was assessed by the intraclass correlation coefficient (ICC) using SPSS (IBM, v.25).
Results
LC–MS/MS
Intracellular, extracellular matrix, and blood-borne proteins were identified by MS. Of the 1413 proteins identified with a high degree of significance, there were 417–617 proteins within each of the five NoCa samples, and 372–691 proteins within each of the five Ca samples (Fig. 2A). There were 142 proteins common in all samples, irrespective of calcification (Fig. 2B). Approximately 26%–41% of the proteins were found to be differentially abundant between Ca and NoCa in the same valve.
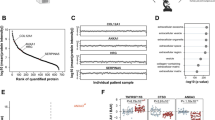
a Venn diagrams show the number of proteins that have been positively identified in the non-calcified and calcified areas within each AV, middle intersections show the number of proteins that are common to both groups. b Venn diagram of the proteins common to calcified and non-calcified areas of the 5 valves. Overlaps show the number of these proteins common between valves (5/5: 142 proteins, 4/5: 49, 3/5: 57). c The 10 pathways found to be most modified between calcified and non-calcified areas according to Ingenuity Pathways Analysis for the 248 proteins found in at least 3 of 5 valves. Blue bars indicate negative activation while orange bars are positive; the color intensity increases with higher absolute activation z-score values
Of the filtered set of 198 proteins found in both the Ca and NoCa regions of at least 3 of 5 valves, the top ten canonical pathways reported by Ingenuity Pathway Analysis are displayed in Fig. 2C. Differences in protein abundance were primarily found in pathways important for the complement-driven immunological response, cellular contraction, cellular signaling, and ECM organization. Out of those pathways, the top upstream regulators identified by IPA were TGFB1 (p = 4.49E-27), dexamethasone (p = 1.48E-24), APP (p = 3.29E-22), MYC (p = 8.35E-22), and lipopolysaccharide (p = 6.84E-20). Based on molecular functions, IPA reported high numbers of proteins involved in the cellular movement (104 proteins, p = 6.65E-7), cell death and survival (127 proteins, p = 8.37E-7), cell-to-cell signaling (92 proteins, p = 1.04E-6), cell maintenance (106 proteins, p = 1.04E-6), and free radical scavenging (35 proteins, p = 5.30E-7). Accompanying clinical chemistry and hematology changes included decreased levels of albumin (p = 8.73E-3), but increased levels of lactate dehydrogenase (LDH; p = 5.55E-3), hematocrit (p = 1.00E-2), alanine aminotransferase (ALT; p = 2.31E-2), and alkaline phosphatase (p = 2.52E-2).
As an alternative means of examining the differences between Ca and NoCa, the top 40 differential proteins selected from the paired Wilcoxon rank analysis of the MS-identified 1413 proteins were presented in a circular heatmap (Fig. 3, also Table 1). These 40 proteins were clustered according to their eight biological functions, namely immunity (the most common functional group with 11 proteins), cell contraction, nucleic acid interaction, metabolism, mineral binding, signaling, angiogenesis, and ECM structural (Fig. 3).
The top 40 differentially present proteins in calcified AV displayed in a heat map for visualization (also listed in Table 1). The proteins were grouped according to their primary biological functions using a representative color scheme on the outermost rim. Each concentric ring represents one individual AV sample. The color spectrum indicates the fold changes of the GAPDH-adjusted emPAI values in calcific samples relative to non-calcific samples, where red represents up-regulation in calcific samples
Osteogenic proteins
Due to the low abundance of osteocalcin, osteonectin, osteopontin (and its isoforms), osteoprotegerin, and RANKL in the MS analysis, their FASTA files protein sequences were searched against the raw MS data. Osteocalcin, osteonectin, and osteopontin (all isoforms) were each identified in only 1 of 5 valves. However, osteoprotegerin was present throughout 4 of the valves, and RANKL was present in NoCa in all 5 values and in the Ca region of just 1 valve.
Using ELISA as an orthogonal, quantitative method to investigate the distribution of osteopontin (OSTP) and osteonectin (SPARC), significantly higher OSTP was found in Ca segments than in NoCa (11.03 ± 2.46 vs. 3.14 ± 1.14 ng/ml, p = 0.0023). In contrast, SPARC tended to be less abundant in Ca than in NoCa (3.59 ± 1.34 vs. 4.46 ± 1.15 ng/ml, p = 0.1602, Fig. 4).
Detection of osteopontin and osteonectin in calcified and non-calcified regions via ELISA. The osteonectin level was lower in the calcified area in 8 out of 10 samples (n = 10, p = 0.1602, paired t-test), whereas the osteopontin level was found to be much higher in the calcified area (n = 10, p = 0.0023, paired t test)
The immunostained tissue sections were evaluated by 2 blinded observers (with high intraclass correlation coefficients) to assess the abundance of osteogenic proteins in calcified, non-calcified, and border regions (Table 2). OSTP, SPARC, OSTCN, and OPG all had the strongest staining in the calcified regions, and RANK staining was greatest in the calcified and border regions. In non-calcified regions, SPARC staining was generally more diffuse but some foci of intense staining were observed (Fig. 5). RANK was also diffusely present in the non-calcified regions whereas it was more localized with the spongiosa in the calcified regions.
Osteopontin (OSTP) positive staining was observed in the collagen-rich fibrosa layer and near the ventricularis surface in the normal tissues (a). In the CAVD tissues (b), positive OSTP staining was found in the calcification sites and in regions immediately adjacent to calcified nodules forming a boundary surrounding the calcific nodule. Occasionally, OSTP staining was seen associated with cell clusters in a metaplasia zone and possibly next to the early calcification sites. Osteonectin (SPARC) staining was more diffuse and loosely scattered in the normal tissues (c). The endothelial lining was also positive for SPARC. However, intense but patchy SPARC staining was observed in the calcification site and in the regions interspersed with cells near calcific nodule (d). Osteocalcin (OSTCN) staining was diffusely distributed in the normal tissue (e). In the CAVD tissue (f), OSTCN-positive staining was observed near the calcific area and associated with fibrous structures of ECM. Occasional, OSTCN was associated with cells in a stratified banding fashion. Osteoprotegerin (OPG/TR11B) was diffusely distributed in the normal tissue (g). OPG was closely associated with calcified areas in CAVD tissues (h), and the staining appeared more intensely in the ventricularis. RANK (TNR11) was faintly distributed throughout the normal tissue (i). RANK staining in CAVD tissues appeared patchy near the calcified areas (j). Negative control stains without primary antibodies are provided. Positive staining is shown by DAB chromogen in brown color. Scale bar = 100 μm
Protein–protein interaction network
The calcification-specific protein network constructed from 24 MS-identified proteins, 5 osteogenic proteins, and 42 additional linker proteins revealed one large interactive network system and three small islands (Fig. 6). A notable cluster within the large network system was complement (C1QA, C1QB, C1QC, C1R, and C1S), which was linked through plasma protease C1 inhibitor (IC1) to SPRC (SPARC). SPRC was in turn connected to CALD1, MMP2, OSTCN, and OSTP and then linked to the rest of the network system. MMP2 was a hub linked to COIA1 and HPLN1, while the actin-related protein 2/3 complex was another cluster linked within the network. The three islands consisted of 1) kallistatin (KAIN), inter-alpha trypsin inhibitor heavy chain H4 (ITIH4), and complement C8 proteins (CO8B, CO8A); 2) the electron transfer flavoproteins ETFA, ETFB; and 3) the ion transporters CYBR1 and FRIL.
Combining the forty MS-identified proteins with the five osteogenic proteins that were validated in the study, the protein interaction information from the String database was used to build a calcification-specific network. The protein network was visualized in Cytoscape, which includes 24 MS-identified proteins (big nodes), 5 osteogenic proteins (big nodes) and 42 additional linker proteins (small nodes) that are known to associate with the key target proteins. The relative difference in protein abundance between the calcified and non-calcified areas are represented by the node color and intensity, while gray represents linker proteins without data. Nomenclature in Table 1
Discussion
This proteomic study delineated the differential proteins involved in CAVD and demonstrated their biological pathways and network interactions. The primary pathways involved in the differential protein data were complement-driven immunological response, acute inflammation, FXR/LXR/RXR/metabolism, cytoskeleton, and ECM organization. Complement and ECM remodeling proteins also were clusters and hubs in the network model, and were connected by the osteogenic proteins, particularly SPARC. Our additional analyses of selected osteogenic proteins confirmed that these five osteogenic proteins were found associated with calcification as indicated by ELISA and IHC scoring.
The main pathways demonstrated by our proteomic analysis were related to immunity and inflammation. Immune and inflammatory cells can infiltrate into the valvular interstitial space and release cytokines/growth factors, promoting endothelial-to-mesenchymal transformation (EndoMT) and ECM remodeling [20]. We previously reported that oxidized lipids, which similarly accumulate in calcified AV tissues, can stimulate VICs to produce mineralized ECM [21]. Here, we identified many proteins in the top-ranked “complement system” and “acute phase response signaling” pathways that were differentially abundant between the Ca and NoCa tissues. Of note, the proteins that collectively form the first complement component (C1QA, C1QB, C1R, C1S) and components of the membrane attack complex (CO8A, CO8B) were generally lower in Ca regions, suggesting that they may have been exhausted due to the chronic stress of calcification. The identification of differentially abundant complement pathway proteins in CAVD confirms the finding from another recent proteomics study [10], as well as in an RNASeq study of human AV endothelial cells under shear stress [22]. Differences between our findings and those previously reported may arise from differences in dissection methods. For example, Schlotter et al., separated the valve leaflets into their 3 layers and also into calcified, fibrotic, and non-calcified regions [10]. In our study, we only dissected the tissue into calcified or non-calcified regions. Thus, fibrotic tissue may be in our calcified regions, further making the results not an exact correspondence. Nonetheless, the finding that the complement pathway was affected in multiple studies of AV proteomics, even if the patterns were not the same, should warrant further investigation of this pathway in CAVD, especially in mechanistic studies.
The calcified tissue segments also showed a differential abundance of several markers for metabolism (Fig. 3), which perform diverse functions ranging from mitochondrial energy production to controlling tissue kallikrein. Correspondingly, the “liver X receptor (LXR)/retinoid X receptor (RXR) activation” and “farnesoid X receptor (FXR)/RXR activation” pathways were highlighted by IPA. The LXR pathway regulates lipid metabolism and inflammation, and LXR activation has been shown to attenuate atherosclerosis in mice [23]. FXR activation has been implicated in the prevention of vascular calcification [24]. Involvement of the LXR and FXR activation pathways is potentially due to the deposition of lipoproteins within the calcific nodules [25]. Since lipid-lowering drugs have not been successful in treating CAVD [20], it may be worth examining LXR and FXR-related aspects of metabolism in CAVD.
The highly dynamic motion and deformation of the heart valve leaflets, which is transmitted by the ECM to the VICs [26], was evident through the differential abundance of proteins related to cell/tissue mechanobiology. The IPA identified “actin cytoskeleton signaling” and the ECM-related “integrin signaling” as the 5th and 7th most involved pathways, respectively. Reductions in the abundance of cytoskeletal actin and actin-binding proteins (i.e., ACTA, CALD1, ARPC3) in the Ca regions may reflect the phenotypic transformation of VICs from quiescent fibroblast to myofibroblast and then to the less contractile osteoblast over the course of CAVD progression [27, 28]. An increase in COEA1 (collagen XIV, a FACIT) and a substantial decrease in HPLN1, hyaluronan and proteoglycan link protein, which is an ECM protein that functions in hyaluronan binding, may reflect an alteration in the interconnectedness of the ECM network in the Ca regions, which would then affect the mechanotransduction to VICs via integrins. In the network model, these ECM proteins were connected to the osteogenic proteins through MMP2. There was also a reduction in OLFL1 (olfactomedin-like 1) in Ca regions; this protein has been reported in the embryonic endocardial cushions and is shown to increase EndoMT in vitro and in vivo [29]. HPLN1 is also believed to contribute to EndoMT [30]. Given that the reactivation of EndoMT has been reported as a contributor to CAVD [31, 32], the reduction in OLFL1 and HPLN1 in these Ca regions suggests that the VICs in the vicinity of large mineralized nodules are post-EndoMT.
Another main category among the top 40 proteins was a group of mineral binding proteins. With the exception of the elevation in FRIL (ferritin light chain), which is involved in iron storage, all of these proteins had lower abundance in the Ca tissue regions. Two of these proteins, AT2B1 and BST1, are involved in Ca2+ signaling/transport, which has been previously linked with aortic stenosis in a GWAS meta-analysis [33]. The gene coding for AT2B1, ARP2B1, has been implicated in coronary artery calcification [34], whereas differential BST1 expression was found in degenerative meniscal cells [10, 35]. Due to the multifaceted roles of calcium, abnormalities in calcium transport and signaling likely merit further attention in investigations of CAVD mechanisms.
Additional differential proteins of interest included 5NDT, a membrane-bound ecto-5’-nucleotidase that converts AMP into adenosine and inorganic phosphate [36]. Mutation of the 5NDT gene was found in persons with peripheral arterial calcification, and cultured fibroblasts from those patients had increased alkaline phosphatase and accumulated intracellular calcium phosphate [37]. HNRPL, a component of heterogeneous nuclear ribonucleoprotein complexes, together with APEX1 regulates the negative calcium responsive element of the APEX promoter [38]. A surprising find was CD109, which has been shown to inhibit TGFβ signaling by direct modulation of receptor activity [39].
We also demonstrated a significant presence of osteogenic proteins within calcified valves. OSTP competes for calcium in/on hydroxyapatite (HA) [40], can bind to various integrins [41], and plays an inhibitory role in vascular calcification [42]. Here, OSTP staining was overt in the perimeter of larger calcified nodules, potentially suggesting an inhibitory role at the nodule boundary. Osteonectin, better known as SPARC, plays an important role in bone calcification and cell proliferation [43], yet here was present in both Ca tissues (focally) and NoCa tissues (diffusely). Osteocalcin (OSTCN) is a vitamin K-dependent ECM protein that binds to HA, and is believed to be a specific product of osteoblasts [44]. It is possible that the OSTCN-associated cells in this CAVD study were newly transformed osteoblast-like cells. Although we have shown these osteogenic proteins to be present in calcified valves, their exact roles remain to be elucidated, especially a potential inhibitory role for the various isoforms of OSTP. Given that SPARC contains TGFβ and Ca2+ binding sites, and was the network hub connection to the complement protein cluster, further investigation is warranted regarding the multiple roles of these osteogenic proteins in driving AV calcification.
These protein differences are intriguing but must be considered in the appropriate context. The human AV tissues used in this study were resected during valve replacement surgeries, and thus are more representative of late-stage CAVD than the early stages of mineralized nodule formation. The non-calcified tissues examined in this study are not representative of normal healthy valve tissue; for example, they may contain micro-calcifications that could not be identified during visual evaluation and dissection. Calcified carotid arteries have been found to have nodules less than 0.1 mm2 [45], and it has been hypothesized that these small calcified particles may coalesce with immediate neighboring micro-calcifications to form the visible calcifications [46]. Proteoglycans and hyaluronan have also been shown to have a differential presence in calcific nodules of aortic valves compared to pre-nodule areas, indicating a potential role for these ECM components in nodule progression [8]. Another limitation is that it was challenging to obtain gender-matched samples since CAVD is more common in men. Sex-related differences in the incidence and presentation of CAVD have raised the question of whether genetic or hormonal effects might shift the mechanism differently [47, 48]. In addition, this study focused on proteins as opposed to gene expression, and may not be entirely consistent with findings from genetic studies due to complex post-transcriptional processing, as well as the potential accumulation of proteins in the valves from elsewhere. Nonetheless, the advantage of studies at the protein level is that the valve function is arguably more a factor of the proteome than the genome.
Conclusion
We report here an array of differential protein profiles in human CAVD, representing diverse biological pathways such as complement-based immune response, acute phase response/inflammation, metabolism, and actin cytoskeletal signaling; many of these pathways were also reported in a comprehensive proteomic analysis of calcified valve leaflets and cultured valve cells [10]. The majority of these differential proteins were connected to the osteogenic proteins in network relationships. These same osteogenic proteins were significantly more abundant in mineralized regions of immunostained human calcified valves. In the future, broader proteomic studies may accelerate the identification of mechanistic details as well as putative biomarkers for the diagnosis and treatment of CAVD.
Abbreviations
- APP:
-
Amyloid-β A4 protein
- a-SMA:
-
A-Smooth muscle actin
- FXR/RXR:
-
Farnesoid X receptor/retinoid X receptor
- LPC:
-
Lysophosphatidylcholine
- LXR/RXR:
-
Liver X receptor/retinoid X receptor
- MAC:
-
Membrane attack complex
- MYC:
-
Myc proto-oncogene protein
- OSTCN*:
-
Osteocalcin
- OSTP*:
-
Osteopontin
- TNR11*:
-
RANK/CD265
- SNP:
-
Single nucleotide polymorphisms
- SPARC*:
-
Osteonectin
- TGFB1:
-
Transformation growth factor-β1
- TR11B*:
-
Osteoprotegerin
References
Demer LL, Tintut Y (2008) Vascular calcification: pathobiology of a multifaceted disease. Circulation 117:2938–2948
Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O’Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM (2011) Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. executive summary: calcific aortic valve disease-2011 update. Circulation 124:1783–1791
Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS (2001) Bone formation and inflammation in cardiac valves. Circulation 103:1522–1528
New SE, Aikawa E (2011) Cardiovascular calcification: an inflammatory disease. Circulation J 75:1305–1313
Martin-Rojas T, Gil-Dones F, Lopez-Almodovar LF, Padial LR, Vivanco F, Barderas MG (2012) Proteomic profile of human aortic stenosis: insights into the degenerative process. J Proteome Res 11:1537–1550
Hinton RB Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE (2006) Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 98:1431–1438
Martin-Rojas T, Mourino-Alvarez L, Alonso-Orgaz S, Rosello-Lleti E, Calvo E, Lopez-Almodovar LF, Rivera M, Padial LR, Lopez JA, de la Cuesta F, Barderas MG (2015) iTRAQ proteomic analysis of extracellular matrix remodeling in aortic valve disease. Sci Rep 5:17290
Stephens EH, Saltarrelli JG, Baggett LS, Nandi I, Kuo JJ, Davis AR, Olmsted-Davis EA, Reardon MJ, Morrisett JD, Grande-Allen KJ (2011) Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc Pathol 20:334–342
Wirrig EE, Hinton RB, Yutzey KE (2011) Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol 50:561–569
Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, Higashi H, DeLaughter DM, Hutcheson JD, Vyas P, Pham T, Rogers MA, Sharma A, Seidman CE, Loscalzo J, Seidman JG, Aikawa M, Singh SA, Aikawa E (2018) Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation 138:377–393
Di Vito A, Donato A, Presta I, Mancuso T, Brunetti FS, Mastroroberto P, Amorosi A, Malara N, Donato G (2021) Extracellular matrix in calcific aortic valve disease: architecture, dynamic and perspectives. Int J Mol Sci 22:913
Gomez-Stallons MV, Tretter JT, Hassel K, Gonzalez-Ramos O, Amofa D, Ollberding NJ, Mazur W, Choo JK, Smith JM, Kereiakes DJ, Yutzey KE (2019) Calcification and extracellular matrix dysregulation in human postmortem and surgical aortic valves. Heart 105:1616–1621
Hutson HN, Marohl T, Anderson M, Eliceiri K, Campagnola P, Masters KS (2016) Calcific aortic valve disease is associated with layer-specific alterations in collagen architecture. PLoS ONE 11:e0163858
Chen JH, Simmons CA (2011) Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res 108:1510–1524
Bini A, Mann KG, Kudryk BJ, Schoen FJ (1999) Noncollagenous bone matrix proteins, calcification, and thrombosis in carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol 19:1852–1861
Shevchenko A, Loboda A, Ens W, Schraven B, Standing KG, Shevchenko A (2001) Archived polyacrylamide gels as a resource for proteome characterization by mass spectrometry. Electrophoresis 22:1194–1203
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19:1639–1645
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ, von Mering C (2017) The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res 45:D362–D368
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Freeman RV, Otto CM (2005) Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111:3316–3326
Wiltz DC, Han RI, Wilson RL, Kumar A, Morrisett JD, Grande-Allen KJ (2014) Differential aortic and mitral valve interstitial cell mineralization and the induction of mineralization by lysophosphatidylcholine in vitro. Cardiovasc Eng Technol 5:371–383
White MP, Theodoris CV, Liu L, Collins WJ, Blue KW, Lee JH, Meng X, Robbins RC, Ivey KN, Srivastava D (2015) NOTCH1 regulates matrix gla protein and calcification gene networks in human valve endothelium. J Mol Cell Cardiol 84:13–23
Calkin AC, Tontonoz P (2010) Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol 30:1513–1518
Miyazaki-Anzai S, Levi M, Kratzer A, Ting TC, Lewis LB, Miyazaki M (2010) Farnesoid X receptor activation prevents the development of vascular calcification in ApoE-/- mice with chronic kidney disease. Circ Res 106:1807–1817
Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R (2007) Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115:377–386
Balachandran K, Sucosky P, Yoganathan AP (2011) Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam 2011:263870
Liu AC, Joag VR, Gotlieb AI (2007) The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171(5):1407–1418
Mohler ER 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH (1999) Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis 8:254–260
Lencinas A, Chhun DC, Dan KP, Ross KD, Hoover EA, Antin PB, Runyan RB (2013) Olfactomedin-1 activity identifies a cell invasion checkpoint during epithelial-mesenchymal transition in the chick embryonic heart. Dis Model Mech 6:632–642
Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A (2007) Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol 310:291–303
Dahal S, Huang P, Murray BT, Mahler GJ (2017) Endothelial to mesenchymal transformation is induced by altered extracellular matrix in aortic valve endothelial cells. J Biomed Mater Res A 105:2729–2741
Wirrig EE, Yutzey KE (2014) Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler Thromb Vasc Biol 34:737–741
Guauque-Olarte S, Messika-Zeitoun D, Droit A, Lamontagne M, Tremblay-Marchand J, Lavoie-Charland E, Gaudreault N, Arsenault BJ, Dube MP, Tardif JC, Body SC, Seidman JG, Boileau C, Mathieu P, Pibarot P, Bosse Y (2015) Calcium signaling pathway genes RUNX2 and CACNA1C are associated with calcific aortic valve disease. Circ Cardiovasc Genet 8:812–822
Ferguson JF, Matthews GJ, Townsend RR, Raj DS, Kanetsky PA, Budoff M, Fischer MJ, Rosas SE, Kanthety R, Rahman M, Master SR, Qasim A, Li M, Mehta NN, Shen H, Mitchell BD, O’Connell JR, Shuldiner AR, Ho WK, Young R, Rasheed A, Danesh J, He J, Kusek JW, Ojo AO, Flack J, Go AS, Gadegbeku CA, Wright JT Jr, Saleheen D, Feldman HI, Rader DJ, Foulkes AS, Reilly MP, Investigators CSP (2013) Candidate gene association study of coronary artery calcification in chronic kidney disease: findings from the CRIC study (Chronic Renal Insufficiency Cohort). J Am Coll Cardiol 62:789–798
Sun Y, Mauerhan DR, Honeycutt PR, Kneisl JS, Norton JH, Hanley EN Jr, Gruber HE (2010) Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord 11:19
Colgan SP, Eltzschig HK, Eckle T, Thompson LF (2006) Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal 2:351–360
St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M (2011) NT5E mutations and arterial calcifications. N Engl J Med 364:432–442
Kuninger DT, Izumi T, Papaconstantinou J, Mitra S (2002) Human AP-endonuclease 1 and hnRNP-L interact with a nCaRE-like repressor element in the AP-endonuclease 1 promoter. Nucleic Acids Res 30:823–829
Finnson KW, Tam BY, Liu K, Marcoux A, Lepage P, Roy S, Bizet AA, Philip A (2006) Identification of CD109 as part of the TGF-beta receptor system in human keratinocytes. FASEB J 20:1525–1527
Hunter GK, O’Young J, Grohe B, Karttunen M, Goldberg HA (2010) The flexible polyelectrolyte hypothesis of protein-biomineral interaction. Langmuir 26:18639–18646
Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS (2001) Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest 107:1055–1061
Speer MY, Chien YC, Quan M, Yang HY, Vali H, McKee MD, Giachelli CM (2005) Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res 66:324–333
Porter PL, Sage EH, Lane TF, Funk SE, Gown AM (1995) Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 43:791–800
Born AK, Lischer S, Maniura-Weber K (2012) Watching osteogenesis: life monitoring of osteogenic differentiation using an osteocalcin reporter. J Cell Biochem 113:313–321
Han RI, Wheeler TM, Lumsden AB, Reardon MJ, Lawrie GM, Grande-Allen KJ, Morrisett JD, Brunner G (2016) Morphometric analysis of calcification and fibrous layer thickness in carotid endarterectomy tissues. Comput Biol Med 70:210–219
Abedin M, Tintut Y, Demer LL (2004) Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol 24:1161–1170
Masjedi S, Lei Y, Patel J, Ferdous Z (2017) Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells. Heart Vessels 32:217–228
Porras AM, McCoy CM, Masters KS (2017) Calcific aortic valve disease: a battle of the sexes. Circ Res 120:604–606
Acknowledgements
The authors thank Katie Brown for blinded IHC scoring. This study was supported in part by NIH grants T32 HL07812 and R21 HL104377.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Reardon serves as a consultant for Medtronic, Boston Scientific, Abbott Medical, and Gore Medical; all fees for such are to his department and there is no overlap between the consulting work and the research presented here.
Ethical standards
Research use of the surgically-resected tissues was approved by the Institutional Review Boards at Houston Methodist Hospital and Rice University and was in accordance with the ethical standards of the IRBs and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained and details that might disclose the identity of patients were removed from samples and the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
In Abbreviations: *Whenever possible, the UniProt (UniProt KB) protein name is used for consistency.
Rights and permissions
About this article
Cite this article
Han, R.I., Hu, C.W., Loose, D.S. et al. Differential proteome profile, biological pathways, and network relationships of osteogenic proteins in calcified human aortic valves. Heart Vessels 37, 347–358 (2022). https://doi.org/10.1007/s00380-021-01975-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01975-z