Abstract
In 2013, a drug-coated balloon catheter (DCB) (SeQuent Please) for the treatment of coronary in-stent restenosis (ISR) was approved in Japan. The pre-marketing Japan domestic NP001 study demonstrated better outcomes of the DCB (n = 138) compared to plain balloon angioplasty (n = 72). After the introduction to marketing, a post-marketing surveillance (PMS) (n = 396) was conducted to evaluate the safety and efficacy of the DCB in Japanese routine clinical practice. The aim of this paper was to assess differences between the pre-marketing NP001 study and the PMS. Compared to the NP001 study, more complex lesions were treated in the PMS (type B2/C: 69.0% vs 20.4%, total occlusion: 11.2% vs 0%, p < 0.001, respectively) and target lesion was more frequently ISR related to drug-eluting stent (DES) (79.5% vs 39.4%, p < 0.001). Regarding clinical outcomes, the rate of target lesion revascularization (TLR) was higher in the PMS than in the NP001 study (TLR: 12.9% at 7 months and 17.6% at 12 months vs 2.8% at 6 months, p = 0.001, p < 0.001, respectively). Multivariable logistic regression analysis revealed that DES-ISR was a risk factor of TLR after DCB treatment for ISR (odds ratio: 5.77, 95% CI 1.75–18.95, p = 0.004). Among representative published trials using DCB for ISR, clinical outcomes are often worse in DES-ISR trials than those in bare metal stent-ISR trials. The rates of TLR in previous DES-ISR trials are similar to that in the current PMS (TLR at 12 months: 22.1% for ISAR-DESIRE 3, 15.3% for PEPCAD-DES, and 13.0% for RIBS IV). The effectiveness and safety of DCB for coronary ISR have been confirmed in the Japanese real-world survey. PMS would be useful to evaluate the safety and effectiveness of medical products throughout their total life cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A clinical trial is the gold standard for evaluating the effectiveness and safety of new medical devices before marketing. Pre-marketing clinical trials are often conducted with a limited number of patients, with specific populations, and in specialized environments. On the other hand, in the real world of medical device use, several factors such as patient and disease characteristics different from those in pre-marketing clinical trials may affect both the effectiveness and safety of medical devices. In Japan, most new innovative medical devices need to be re-examined during a certain time period after their introduction into the market according to the post-marketing surveillance (PMS) system under the Pharmaceuticals and Medical Devices Act [1,2,3]. To ensure the effectiveness, safety, and quality of medical devices after their approval for marketing, PMS is conducted under the good post-marketing surveillance practice (GPSP), which is the Japanese system for ensuring the quality and reliability of the survey [4]. Following regulatory re-evaluation of the PMS, the results are classified into one of three approval categories: (1) approval refused (manufacturing and marketing suspended, approval revoked), (2) changes in approval (modifications in approved items as directed), and (3) approved (as per application for re-examination), and any additional required risk management measures are adopted [5].
In July 2013, the first drug-coated balloon catheter (DCB) (SeQuent Please, B Braun, Melsungen, Germany, and Nipro Corporation, Osaka, Japan) for the treatment of coronary in-stent restenosis (ISR) was approved in Japan. In the pre-marketing stage, the Japan domestic randomized clinical trial (NP001 study) demonstrated that compared to plain old balloon angioplasty (POBA), the clinical outcome of the DCB for ISR was more favorable at 6 months follow-up [6]. At the time of approval of the DCB, the company that developed the device was required to conduct a PMS to evaluate the safety and efficacy of the DCB in Japanese routine clinical practice. In the regulatory review of the PMS results, the clinical outcome of the DCB in the PMS differed markedly from those reported in the NP001 study [7].
In this paper, we, the Pharmaceuticals and Medical Devices Agency (PMDA): the Japanese regulatory agency, compared baseline characteristics and clinical outcomes between the pre- and post-marketing clinical studies involving the use of the DCB for ISR and evaluated risk factors for worse clinical outcome after the DCB treatment for ISR lesions. In addition, we presented the Japanese regulatory view on the DCB PMS results.
All analyses were performed using data from application dossiers for the regulatory evaluation. This paper is not written for promotional purposes and the views expressed do not necessarily represent the views and findings of the PMDA. The protocol of the study was approved by the PMDA IRB (certification number: 2019-Z-10), and followed the Declaration of Helsinki and the ethical standards of the responsible committee on human experimentation.
Materials and methods
Outline of clinical studies
Pre-marketing clinical study for approval in Japan (NP001 study)
Between October 2009 and October 2011, 210 patients were enrolled from 13 centers across Japan and were randomized into the DCB group (n = 138) and the POBA group (n = 72). These patients underwent percutaneous coronary intervention (PCI) with DCB or POBA for ISR lesions. The main exclusion criteria were the occurrence of acute myocardial infarction (MI) within the preceding 72 h, the presence of severe renal insufficiency, and the presence of severe liver disease. Major angiographic exclusion criteria included the presence of lesions longer than 22 mm, vessels with diameters below 2.0 mm, the presence of total occlusion, unprotected left main stenosis, and a bifurcation lesion treated using the kissing balloon technique, vessels with previous paclitaxel-eluting stent implantation, and vessels treated with drug-eluting stent (DES) within 24 weeks. The primary endpoint was target vessel failure (TVF) at 6 months after PCI. TVF was defined as the occurrence of cardiac death, myocardial infarction, or target vessel revascularization (TVR). TVR or target lesion revascularization (TLR) was defined as either revascularization at the target lesion by PCI or coronary artery bypass grafting (CABG). Major adverse cardiac events (MACE) were defined as a composite of all-cause death, MI, and TVR. All patients provided written informed consent. During the follow-up, a patient was excluded due to the revocation of consent. This study was sponsored by the Nipro Corporation.
Post-marketing surveillance in Japan
The DCB PMS is a prospective, single arm, nationwide surveillance. Between January 2014 and July 2016, 396 patients were enrolled from 53 centers across Japan. These patients underwent PCI with DCB for ISR lesions. There were no specific exclusion criteria. Patients were scheduled for an angiographic follow-up after 7 months and a 12-month clinical follow-up after PCI. The definitions of TVF, TVR, TLR, and MACE were the same as those used in the NP001 study. In 12 patients, both clinical and angiographic follow-up were not performed due to the intention of each patient. The PMS was funded and conducted by the Nipro Corporation under the GPSP Ordinance of the Japanese Ministry of Health, Labor, and Welfare. Informed consent from individual patients was waived, because post-marketing surveillance is legally obligated to marketing authorization holder under the provision of the Pharmaceuticals and Medical Devices Act in Japan.
Statistical analysis
Continuous variables are presented as the means with standard deviations. Differences in continuous parameters were evaluated using an unpaired t test. Categorical variables are presented as frequency counts and intergroup comparisons were made using Fisher’s exact test or Chi-square test. To identify risk factors for TLR following DCB treatment for ISR, a multivariable logistic regression model was constructed using patient-level clinical data from the PMS. Independent variables were chosen as potentially significant independent factors with a p value level < 0.10 by univariate analysis. The final model was obtained using a forward stepwise method with a threshold for exit set at a p value level > 0.10. Factors in each comparison were expressed as odds ratios (ORs) and their 95% confidence intervals (CIs). Statistical analysis was performed using SPSS Statistics software (version 22.0, SPSS Inc., Chicago, IL). A p value < 0.05 was considered statistically significant.
Results
Differences between the pre- and post-marketing clinical studies
Baseline characteristics
The comparison of baseline characteristics between the NP001 study and the PMS is shown in Table 1. At baseline, more patients in the PMS had heart failure, peripheral artery disease, and chronic kidney disease compared to the NP001 study, whereas the prevalence of prior MI, prior stroke and hyperlipidemia was higher in the NP001 study. In the PMS, 13.4% of patients received hemodialysis that was one of the exclusion criteria in the NP001 study. Regarding lesion characteristics, more complex lesions were treated with DCB in the PMS (type B2/C lesion: 69.0% vs 20.4%, p < 0.001, Mehran ISR classification [8] type IV: total occlusion lesion: 11.2% vs 0%, p < 0.001). In addition, approximately 80% of all lesions in the PMS were ISR lesions that occurred after DES implantation, whereas the proportion of DES-ISR was around 40% in the NP001 study (the proportion of DES-ISR: 79.5% vs 39.4%, p < 0.001).
Clinical and angiographic outcomes
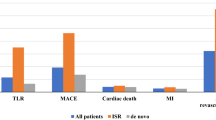
Clinical and angiographic outcomes of the two studies are summarized in Table 2. The rate of MACE, TVF, TVR and TLR was significantly higher in the PMS than in the NP001 study, respectively, (PMS: MACE 16.9% at 7 months and 27.1% at 12 months, TVF 16.1% at 7 months and 24.7% at 12 months, TVR 14.9% at 7 months and 22.1% at 12 months, TLR 12.9% at 7 months and 17.6% at 12 months; NP001 study: MACE 6.6% at 6 months, TVF: 6.6% at 6 months, TVR 6.4% at 6 months, TLR: 2.8% at 6 months).
Quantitative coronary angiography (QCA) analysis revealed that the diameter stenosis (DS), binary restenosis (BR) rate, and late lumen loss (LLL) during the follow-up period was significantly higher in the PMS than in the NP001 study (DS: 41.6% vs 28.1%, BR: 27.2% vs 4.4%, and LLL: 0.41 mm vs 0.11 mm, respectively).
Risk factors for TLR following DCB treatment for ISR
Multivariate logistic regression analysis revealed that DES-ISR was a risk factor for TLR at 12 months after PCI using DCB for ISR (OR: 5.77, 95% CI 1.75–18.95, p = 0.004) (Table 3).
Discussion
The current main findings were: (1) patient background and lesion characteristics in the PMS differed from those in the pre-marketing NP001 study, (2) the rate of TLR in the PMS was markedly higher than in the NP001 study and (3) multivariate analysis revealed that DES-ISR was a risk factor for TLR after PCI using DCB for ISR.
In the NP001 study, the study protocol described that vessels with previous paclitaxel-eluting stent implantation and vessels treated with other DES within 24 weeks were excluded due to safety concern such as drug overdose at that time. Consequently, the proportion of DES-ISR in the NP001 study was much smaller than in the PMS. Some reports have shown that DES-ISR is different from bare metal stents (BMS)-ISR in terms of its mechanism of action, morphological patterns, tissue composition, and response to treatment [9,10,11,12]. Clinical outcomes in representative published trials that used DCB for ISR are summarized in Table 4. Among these trials, TLR and MACE occurred more often in the trials that involved DES-ISR [13,14,15] than in the trials that involved BMS-ISR [16,17,18]. The results of the DES-ISR trials were similar to the result of the current PMS (TLR at 12 months: 22.1% for ISAR-DESIRE 3 [13], 15.3% for PEPCAD-DES [14], and 13.0% for RIBS IV [15]). Therefore, the marked differences in clinical outcomes between the NP001 study and the PMS could be mainly attributed to the difference in the proportion of DES-ISR lesions in each study.
There are several treatment options for ISR including DCB, repeat stenting with DES, and POBA. Recent guidelines in Japan and the European Union recommend both DCB and DES treatments for ISR as class I [19, 20]. Previous trials have shown that repeat stenting with DES is effective and safe in patients with ISR [13, 15, 17, 18, 21, 22]. However, in repeat DES stenting, there is a concern about prolonged dual antiplatelet therapy (DAPT) duration due to the additional metal layers in the coronary vessel. In this respect, DCB treatment can offer the benefit of drug delivery to the ISR site without additional metal layers [23, 24], which could lead to minimization of the DAPT duration.
Considering all the above, the PMDA concluded that the benefit of DCB treatment for ISR outweighs its risk and that DCB could be an effective and safe option for the treatment of coronary ISR lesions. Finally, the DCB-PMS result was classified as “approved (as per application for re-examination)”.
Pre-marketing clinical trials are often conducted with specific populations and in specialized environments because of control variability, data quality and cost. However, the populations enrolled in the trials may differed from those seen in routine practice. In the regulatory approval review of medical products, generalizability is always be discussed. Consequently, the indication and/or target patients of products is not always the same as in the pre-marketing trials. Regarding this point, PMS would be very useful and effective system to determine effectiveness and safety of medical products in the real-world practice. As the target population or the proportion of specific lesions such as complex lesions are sometimes largely different between pre-marketing clinical trials and PMS, unexpected results could be obtained in the PMS. In such a situation, a cause analysis is often performed. A cause analysis is very useful in optimizing the benefit–risk balance of medical devices. However, there are some limitations in the analysis of PMS, such as the unavailability of an active control, limited follow-up period, and data resource limitations. In the current case, some published data are supportive of the PMS result. Although PMS have some limitations compared to controlled pre-marketing clinical trials, PMS could be useful to evaluate the safety and effectiveness of medical devices throughout their total life cycles.
Limitations
Some limitations should be noted. First, there was limited information concerning the results of laboratory investigations, echocardiographic findings and medications in the PMS. Second, 12-month follow-up data of the NP001 study are unavailable because the follow-up period ends 6 months. Third, the current PMS was a single arm surveillance.
Therefore, significant advantages of DCB treatment over repeat DES stenting in patients with DES-ISR remain unknown. A large-scale randomized trial comparing DCB and repeat DES stenting for DES-ISR in Japanese routine clinical practice, would be warranted.
Conclusions
The effectiveness and safety of DCB for coronary ISR have been confirmed in the Japanese real-world survey. In the real-world, the target population and/or lesions of medical products is not always the same as in the pre-marketing trials. Regarding this point, PMS would be useful to evaluate the safety and effectiveness of medical products throughout their total life cycles.
Abbreviations
- BMS:
-
Bare metal stents
- BR:
-
Binary restenosis
- CABG:
-
Coronary artery bypass grafting
- CI:
-
Confidence intervals
- DAPT:
-
Dual antiplatelet therapy
- DCB:
-
Drug-coated balloon
- DES:
-
Drug-eluting stents
- DS:
-
Diameter stenosis
- GPSP:
-
Good post-marketing surveillance practice
- ISR:
-
In-stent restenosis
- LAD:
-
Left anterior descending artery
- LCX:
-
Left circumflex artery
- LMT:
-
Left main trunk
- LLL:
-
Late lumen loss
- MACE:
-
Major adverse cardiac events
- MI:
-
Myocardial infarction
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
- PMDA:
-
Pharmaceuticals and Medical Devices Agency
- PMS:
-
Post-marketing surveillance
- POBA:
-
Plain old balloon angioplasty
- QCA:
-
Quantitative coronary angiography
- RCA:
-
Right coronary artery
- RVD:
-
Reference vessel diameter
- TLR:
-
Target lesion revascularization
- TVF:
-
Target vessel failure
- TVR:
-
Target vessel revascularization
References
Pharmaceuticals and Medical Devices Agency. Use-results survey system under the pharmaceuticals and medical devices act. https://www.pmda.go.jp/files/000160083.pdf. Accessed 17 Mar 2020. (in Japanese)
Pharmaceuticals and Medical Devices Agency. Fundamental point of view about use-results survey system. https://www.pmda.go.jp/files/000197726.pdf. Accessed 17 Mar 2020. (in Japanese)
Konishi A, Ho M, Shirai Y, Shirato H (2018) First approval of improved medical device conditional on use-result survey in Japan—regulatory review of polymer-free drug-coated biofreedom coronary stent. Circ J 82:1487–1490
Konishi A, Ho M, Ouchi T, Mitsutake Y, Shirato H (2019) Regulatory approval review of transcatheter mitral valve repair—difference in the indication between the USA and Japan. J Cardiol 74:13–18
Pharmaceuticals and Medical Devices Agency. Re-examinations. https://www.pmda.go.jp/review-services/reexamine-reevaluate/re-evaluations/0009.html. Accessed 17 Mar 2020. (in Japanese)
Review Report: SeQuent Please drug eluting balloon. http://www.pmda.go.jp/medical_devices/2013/M201300030/530100000_22500BZX00322000_A100_1.pdf. Accessed 17 Mar 2020. (in Japanese)
Re-evaluation Report: SeQuent Please drug eluting balloon. https://www.pmda.go.jp/medical_devices_reexam/2020/M20200319001/530100000_22500BZX00322000_100_1.pdf. Accessed 25 Mar 2020. (in Japanese)
Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB (1999) Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation 100:1872–1878
Lemos PA, van Mieghem CA, Arampatzis CA, Hoye A, Ong AT, McFadden E, Sianos G, van der Giessen WJ, de Feyter PJ, van Domburg RT, Serruys PW (2004) Post-sirolimus-eluting stent restenosis treated with repeat percutaneous intervention: late angiographic and clinical outcomes. Circulation 109:2500–2502
Mishkel GJ, Moore AL, Markwell S, Shelton MC, Shelton ME (2007) Long-term outcomes after management of restenosis or thrombosis of drug-eluting stents. J Am Coll Cardiol 49:181–184
Steinberg DH, Gaglia MA Jr, Pinto Slottow TL, Roy P, Bonello L, De Labriolle A, Lemesle G, Torguson R, Kineshige K, Xue Z, Suddath WO, Kent KM, Satler LF, Pichard AD, Lindsay J, Waksman R (2009) Outcome differences with the use of drug-eluting stents for the treatment of in-stent restenosis of bare-metal stents versus drug-eluting stents. Am J Cardiol 103:491–495
Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schömig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabaté M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Jüni P (2007) Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 370:937–948
Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, Schulz S, Fusaro M, Ott I, Ibrahim T, Hausleiter J, Valina C, Pache J, Laugwitz KL, Massberg S, Kastrati A, ISAR-DESIRE 3 investigators (2013) Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet 381:461–467
Rittger H, Waliszewski M, Brachmann J, Hohenforst-Schmidt W, Ohlow M, Brugger A, Thiele H, Birkemeyer R, Kurowski V, Schlundt C, Zimmermann S, Lonke S, von Cranach M, Markovic S, Daniel WG, Achenbach S, Wöhrle J (2015) Long-term outcomes after treatment with a paclitaxel-coated balloon versus balloon angioplasty: insights from the PEPCAD-DES Study (Treatment of Drug-eluting Stent [DES] In-Stent Restenosis With SeQuent Please Paclitaxel-Coated Percutaneous Transluminal Coronary Angioplasty [PTCA] Catheter). JACC Cardiovasc Interv 8:1695–1700
Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, García-Touchard A, López-Minguéz JR, Benedicto A, Masotti M, Zueco J, Iñiguez A, Velázquez M, Moreno R, Mainar V, Domínguez A, Pomar F, Melgares R, Rivero F, Jiménez-Quevedo P, Gonzalo N, Fernández C, Macaya C, RIBS IV Study Investigators (under auspices of Interventional Cardiology Working Group of Spanish Society of Cardiology) (2015) A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV Randomized Clinical Trial. J Am Coll Cardiol 66:23–33
Scheller B, Clever YP, Kelsch B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Speck U, Böhm M, Cremers B (2012) Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv 5:323–330
Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, Werner GS, Antoni D, Kleber FX, Bocksch W, Leschke M, Ackermann H, Boxberger M, Speck U, Degenhardt R, Scheller B (2015) Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: the three-year results of the PEPCAD II ISR study. EuroIntervention 11:926–934
Alfonso F, Pérez-Vizcayno MJ, García Del Blanco B, Otaegui I, Masotti M, Zueco J, Veláquez M, Sanchís J, García-Touchard A, Lázaro-García R, Moreu J, Bethencourt A, Cuesta J, Rivero F, Cárdenas A, Gonzalo N, Jiménez-Quevedo P, Fernández C, RIBS V Study Investigators (2016) Long-term results of everolimus-eluting stents versus drug-eluting balloons in patients with bare-metal in-stent restenosis: 3-year follow-up of the RIBS V Clinical Trial. JACC Cardiovasc Interv 9:1246–1255
JCS 2018 Guideline on Revascularization of Stable Coronary Artery Disease. http://j-circ.or.jp/guideline/. Accessed 17 Mar 2020. (in Japanese)
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40:87–165
Kang IS, Shehata I, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK (2016) Comparison between drug-coated balloon angioplasty and second-generation drug-eluting stent placement for the treatment of in-stent restenosis after drug-eluting stent implantation. Heart Vessels 31:1405–1411
Sakamoto Y, Yamawaki M, Araki M, Kobayashi N, Mori S, Tsutsumi M, Honda Y, Hirano K, Ito Y (2019) Comparison of 12-month angiographic outcomes between repeat drug-eluting stent implantation and drug-coated balloon treatment for restenotic lesion caused by stent fracture. Heart Vessels 34:1589–1594
Akutsu N, Ogaku A, Koyama Y, Fujito H, Ebuchi Y, Migita S, Morikawa T, Tamaki T, Mineki T, Kougo T, Kojima K, Iida K, Murata N, Nishida T, Oshima T, Sudo M, Kitano D, Haruta H, Fukamachi D, Takayama T, Hiro T, Hirayama A, Okumura Y (2019) Effect of drug-coated balloon angioplasty on in-stent restenotic coronary lesions analyzed with optical coherence tomography and serial coronary artery angioscopy. Heart Vessels 34:1925–1935
Her AY, Shin ES, Chung JH, Kim YH, Garg S, Lee JM, Doh JH, Nam CW, Koo BK (2019) Plaque modification and stabilization after paclitaxel-coated balloon treatment for de novo coronary lesions. Heart Vessels 34:1113–1121
Acknowledgments
We thank the members of the PMDA review team for their input and Editage (https://www.editage.com) for English editing. The views expressed in this article are those of the authors and do not necessarily reflect the official views of the PMDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. This paper was written with no external funding.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitsutake, Y., Konishi, A., Shiba, T. et al. Differences in clinical outcomes between pre- and post-marketing clinical study following paclitaxel-coated balloon catheter treatment for coronary in-stent restenosis: from the Japanese regulatory viewpoint. Heart Vessels 36, 155–162 (2021). https://doi.org/10.1007/s00380-020-01676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01676-z