Abstract
Diastolic intraventricular pressure difference (IVPD) reflects left ventricular (LV) diastolic function. The relative pressure imaging (RPI) enables the noninvasive quantification of IVPD based on vector flow mapping (VFM) and visualization of regional pressure distribution. LV dyssynchrony causes deterioration of cardiac performance. However, it remains unclear how IVPD is modulated by LV dyssynchrony. LV dyssynchrony was created in ten open-chest dogs by right ventricular (RV) pacing. The other ten dogs undergoing right atrial (RA) pacing set at the similar heart rate with RV pacing were used as controls. Echocardiographic images were acquired at baseline and during pacing simultaneously with LV pressure measurement by a micromanometer. Pressure difference (ΔP) was computed between the apex and the base of the LV inflow tract during a cardiac cycle by RPI and ΔP during isovolumic relaxation time (ΔPIRT), a parameter of diastolic suction, and that during early filling phase (ΔPE) were measured. During RV pacing, stroke volume (SV) and ΔPIRT decreased significantly, while ΔPE did not change compared to the baseline. During RA pacing, SV, ΔPIRT and ΔPE did not change significantly. ΔPIRT tended to correlate with –dP/dtmin and end-systolic volume, and significantly correlated with ejection fraction. IVPD during isovolumic relaxation time was decreased by LV dyssynchrony, while IVPD during early filling phase was not. A reduction of diastolic suction is observed in LV dyssynchrony and is significantly related to a decrease in SV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraventricular pressure difference (IVPD) exists in the left ventricle (LV) through a cardiac cycle and it has been shown to be associated with cardiac function [1,2,3]. IVPD during early diastole has been reported to reflect LV diastolic suction as well as diastolic filling [4,5,6]. Since suction plays an important role in promoting early rapid filling and accepting adequate filling volume under low filling pressure, IVPD may be an additional marker to assess diastolic suction and diagnose diastolic dysfunction. As a noninvasive method of evaluating IVPD, color M-mode Doppler (CMMD) echocardiography integrating the Euler equation has been proposed [7]. However, this method is applied only to a single direction, parallel to the beam path.
Vector flow mapping (VFM®, Hitachi) is a new echocardiographic technology to evaluate intracardiac flow velocity vectors. It can assess flow velocity in the direction not only parallel but also perpendicular to the beam based on the combination of the continuity equation and speckle tracking echocardiography [8]. The velocity information can be converted to the pressure information using the Navier–Stokes equation (NSE) and noninvasive assessment of intraventricular two-dimensional distribution of relative pressures is now feasible with a software developed by Hitachi (Relative Pressure Imaging (RPI), Hitachi Co.) [9].
Some patients with heart failure have LV dyssynchrony accompanying electrical conduction abnormalities such as left bundle branch block (LBBB) [10]. Inefficient LV work due to dyssynchronous wall motion causes deterioration of cardiac performance and results in the adverse prognosis [11]. Dyssynchronous LV contraction affects the following relaxation [10]. However, it remains unclear how diastolic IVPD is modulated by LV dyssynchrony.
In the present study, we analyzed IVPD noninvasively using RPI before and during LV dyssynchrony induced by right ventricular (RV) pacing and investigated the mechanism of impaired diastolic function in LV dyssynchrony.
Methods
Animal preparation
Our experimental study was approved and conducted in accordance with the rules of the animal experiments committee of Osaka University. Twenty open-chest dogs (female, 9.9 ± 0.7 kg) were used in this study. After intramuscular injection of xylazine hydrochloride (0.5 mg/kg), the dogs were anaesthetized by pentobarbital sodium (25.9 mg/kg) administered via a peripheral vein and then were intubated and ventilated with room air and oxygen using a Harvard respirator (SN-480–3, Shinano, Tokyo, Japan). Oxygen saturation and electrocardiogram were continuously monitored. After induction of anesthesia, the dogs were placed in supine position, and continuous intravenous infusion of lactated Ringer’s solution was administered via a peripheral vein. Anesthesia was maintained through continuous infusion of pentobarbital sodium (6 mg/kg/h) and midazolam (0.18 mg/kg/h). Buprenorphine (4 μg/kg) was administered intramuscularly for analgesia. Pancuronium bromide (1.0 mg) was administered intravenously as a muscle relaxant. The chest was opened through a thoracotomy in the longitudinal direction and the heart was suspended in a pericardial cradle.
A 6-Fr micromanometer catheter (Millar Instruments, Houston, Texas) was inserted into the LV through the right femoral artery or right common carotid artery to measure pressure. LV systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), maximum and minimum time derivatives of LV pressure (dP/dtmax, dP/dtmin) and time constant of LV relaxation (tau) were averaged for five consecutive beats. Tau was calculated assuming non-zero asymptote in the data from the point of the minimum time derivatives of the LV pressure to the point at which pressure decreased to the level of the LVEDP.
Echocardiography
The ultrasound equipment was Prosound F75 (Hitachi, Ltd., Tokyo, Japan). Apical long-axis color Doppler images were obtained using a UST-52124 probe, at a transducer frequency of 5 MHz, depth of 7 cm, and frame rate of 35–46 frames/second. The timings of aortic valve closure (AVC) and mitral valve opening (MVO) were determined from LV outflow tract (LVOT) flow and transmitral flow images obtained using pulsed wave Doppler echocardiography. Stroke volume (SV) was calculated using velocity–time integral of LVOT flow and LVOT diameter. Ejection fraction (EF) was calculated at the apical four-chamber view by the simplified Simpson’s formula. Echocardiographic images were captured over five successive beats and echocardiographic parameters were averaged for three successive beats.
Experimental protocol
LV dyssynchrony was induced by RV pacing in ten dogs using a 4F pacing catheter connected to the external pulse generator (Canute, Welwyn Garden, UK). This pacing catheter was inserted into the RV from the right femoral vein, using echocardiographic imaging as a guide. We confirmed the creation of LV dyssynchrony by prolonged QRS duration by the electrocardiogram and dyssynchronous wall motion by two-dimensional echocardiography. We also measured time difference (TD) between the first peaks of longitudinal strain at the apical anterior and mid inferolateral segments. As a control with heart rate similar to RV pacing, right atrial (RA) pacing was performed in the other ten dogs using the electrode attached to the RA appendage. Each pacing rate was set at about 150 ppm (151 ± 2 ppm) and a stable condition was ensured. Echocardiographic images, heart rate and LV pressure were acquired at baseline and during each pacing.
Image analysis
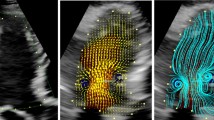
RPI, a new noninvasive method to calculate intracardiac pressure distribution, uses velocity field of VFM and the momentum equation. Intraventricular flow velocity vectors of VFM are computed by solving the continuity equation using flow velocity obtained from color Doppler and wall velocity obtained from speckle tracking echocardiography [12, 13]. RPI method converts this velocity information to relative pressure distribution using the momentum equation of fluid motion named NSE (1) (Fig. 1).
Relative pressure imaging. Velocity information of VFM can be converted to pressure information based on the Navier–Stokes equation. a Yellow arrows represent regional flow velocity vectors of VFM. b The color map reflects relative pressure distribution. Warm color indicates higher and cold color indicates lower pressure relative to reference point that is automatically set at the center of the region of interest
We use two-dimensional NSE by omitting terms related to the third direction because velocity field of VFM is two dimensional. To reduce the size of the system of equations, the divergence operation is applied to the NSE [9]. Pressure distribution is visualized by describing higher pressure as warm color and lower pressure as cold color relative to a reference point. The reference point has a pressure value of 0 mmHg and is automatically set at the center of the region of interest.
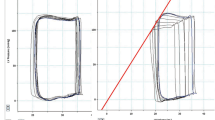
In this study, color Doppler apical long-axis images were analyzed offline by using VFM analysis software (DAS-RS 1, Hitachi, Ltd.). The endocardial border was manually traced at end-diastole and then automatically determined throughout the remaining frames. The wall velocity was determined by speckle tracking echocardiography and then intraventricular pressure distribution was visualized. By setting a sampling line in the region of interest, a pressure difference between both ends of the line is calculated automatically. The pressure difference between the apex and the base of the LV inflow tract (ΔP) was computed during a cardiac cycle and peak ΔP during isovolumic relaxation time (ΔPIRT) and that during early filling phase (ΔPE) were measured (Fig. 2). These IVPD parameters were averaged for three consecutive cardiac cycles.
Measurement of IVPD. a By setting a sampling line (yellow line) in the region of interest, a pressure difference between both ends of the line is calculated automatically. In the present study, pressure difference (ΔP) was computed between the apex and the base of the LV inflow tract frame by frame during a cardiac cycle. b ΔP profile during one cardiac cycle. The negative value means the apical pressure is lower than the basal pressure. ΔP during isovolumic relaxation time (ΔPIRT) and that during early filling phase (ΔPE) were measured. AVC aortic valve closure; MVO mitral valve opening
Statistical analysis
All values were shown as mean ± SD. Baseline and pacing conditions were statistically compared using paired t tests. RV pacing and RA pacing conditions were statistically compared using unpaired t tests. The Pearson product–moment correlation coefficient was used to identify relationships between selected variables. Significance level was set at P < 0.05.
Results
Hemodynamics and echocardiographic data
Hemodynamics and echocardiographic data at baseline and during pacing are shown in (Table 1). Heart rate increased and LVEDP decreased significantly by RV and RA pacing. LVSP, dP/dtmax, dP/dtmin, SV and EF significantly decreased by RV pacing. Prolongation of TD was consistent with successful creation of LV dyssynchrony with RV pacing.
Relative pressure imaging
Figure 3 shows representative ΔP profiles at baseline and during each pacing and (Table 2) shows changes in ΔPIRT and ΔPE. During RV pacing, ΔPIRT decreased significantly, while ΔPE did not change compared to the baseline. During RA pacing, ΔPIRT and ΔPE did not change significantly. Representative relative pressure images during a cardiac cycle are shown in (Fig. 4). Relative pressure images during isovolumic relaxation time showed IVPD during RV pacing was lower than that at baseline and during RA pacing. Interestingly, in 8 out of 10 dogs during RV pacing, accompanying early septal contraction, a significant pressure gradient between the posterior area and the septal area (warmer color in the septal area indicating posterior pressure < septal pressure) was detected at early systole, while in all dogs at baseline and during RA pacing the significant horizontal pressure gradient was not detected at early systole.
Representative ΔP profiles at baseline and during each pacing. The Y axis indicates pressure difference (ΔP) between the apex and the base of the inflow tract, and the X axis indicates R–R interval. During RV pacing, ΔPIRT decreased while ΔPE did not change. During RA pacing, ΔPIRT and ΔPE did not change. AVC aortic valve closure; MVO mitral valve opening
Representative relative pressure images during a cardiac cycle. Warm color indicates higher and cold color indicates lower pressure relative to the reference point. Images during isovolumic relaxation time showed lower IVPD during RV pacing compared with baseline and RA pacing. The pressure gradient from the posterior to the septal side (warmer color at the septal side) accompanying early septal contraction existed during RV pacing. AVC aortic valve closure; MVO mitral valve opening
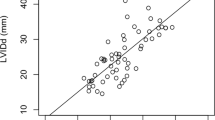
Correlation between ΔPIRT and systolic or diastolic parameters
ΔPIRT correlated significantly with EF (r = − 0.52, P = 0.02). Furthermore, ΔPIRT tended to correlate with dp/dtmin (r = 0.40, P = 0.09) and tau (r = 0.42, P = 0.11), although these did not reach statistical significance. ΔPIRT did not correlate with dP/dtmax (Fig. 5).
Relationships between ΔPIRT and systolic and diastolic parameters. The black circles indicate the data obtained during RV pacing and the white circles indicate the data obtained during RA pacing. A significant correlation was found between ΔPIRT and EF. dP/dtmax maximum time derivative of left ventricular pressure; dP/dtmin minimum time derivative of left ventricular pressure; EF, LV ejection fraction; tau, time constant of LV relaxation
Discussion
In the present study, we investigated how IVPD was changed by LV dyssynchrony using VFM. IVPD during isovolumic relaxation time was decreased by LV dyssynchrony induced by RV pacing. In contrast, in RA pacing it was not changed. IVPD during isovolumic relaxation time was associated with EF.
Measurement of IVPD
Noninvasive estimation of IVPD based on CMMD echocardiography is a well-validated technique [1, 5, 14]. However, this method can assess only pressure difference along a straight scan line. RPI is a novel method based on velocity vectors obtained by VFM and the NSE [9]. In addition to measurement of a pressure difference, it allows visualization of intraventricular pressure distribution. Thus, RPI may reveal how pressure distribution is modulated by various heart diseases. In the present study, the decrease in intraventricular pressure gradient during isovolumic relaxation time in LV dyssynchrony was detected visually as shown in (Fig. 4). In addition, the pressure gradient from the posterior to the septal side was observed accompanying dyssynchronous early septal contraction. It is considered that remarkable pressure gradient in the horizontal direction would not be generated if all LV segments contract in a synchronous manner. Therefore, the posterior to septal pressure gradient may be caused by dyssynchronous wall motion.
Previous studies have focused on IVPD during early filling phase mainly [5, 7]. However, we focused on IVPD during not only early filling phase but also isovolumic relaxation time. During isovolumic relaxation time, although both the aortic valve and mitral valve are closed, apically directed flow have been detected in the intact LV with CMMD and pulsed wave Doppler echocardiography [15, 16]. That proves pressure difference between LV apex and base exists during isovolumic relaxation and this IVPD is considered as the force to redistribute the blood in the LV and promote early diastolic filling [15]. We considered IVPD during isovolumic relaxation time should reflect LV suction and provide valuable insight into LV diastolic and systolic function. Yotti et al. defined peak IVPD that was reached during isovolumic relaxation time, just after AVC as reverse ejection IVPD. They showed reverse ejection IVPD reflected the rate of LV relaxation.1 Furthermore, Nagomi et al. showed that kinetic energy of isovolumic relaxation flow calculated by VFM associated with systolic function, LV geometry and untwisting mechanics [6].
IVPD in LV dyssynchrony
RV pacing induces an abnormal activation sequence and dyssynchronous ventricular contraction comparable with LBBB. Subsequently, dyssynchronous LV relaxation also occurs. A previous study demonstrated dyssynchronous strain curve in diastole in patients with LBBB and with RV pacing [10]. Using CMMD echocardiography, Lin et al. observed that apically directed flow during isovolumic relaxation time was disturbed by RV apical pacing. They considered that it reflected the disruption of intraventricular pressure gradient during isovolumic relaxation time [16]. In the present study, IVPD during isovolumic relaxation time was decreased by LV dyssynchrony. This showed apical LV pressure was hard to decrease during isovolumic relaxation time due to abnormal LV relaxation following dyssynchronous contraction.
LV suction relates closely with systolic function because the elastic recoil plays an important role in generating LV sucking effect [17]. When the LV contracts below its equilibrium volume, the potential energy is stored during systole. Due to release of potential energy during early diastole, LV relaxation is promoted [18]. Yanada et al. indicated that velocity of apically directed isovolumic relaxation flow correlated with EF and early diastolic propagation velocity, and concluded isovolumic relaxation flow played a role in delivering good LV systolic performance to LV early diastolic filling [15]. Nakatani et al. showed the peak negative LV pressure during mitral occlusion which was measured as an index of suction strongly and negatively correlated with EF and tended to correlate with dP/dtmin in the human ventricle [17]. In the present study, IVPD during isovolumic relaxation time correlated with EF moderately and negatively, and tended to correlate with dP/dtmin and tau comparable with those previous studies. In addition to dyssynchronous LV relaxation, reduction of elastic recoil due to systolic dysfunction may also induce reduction of IVPD during isovolumic relaxation time leading to a decrease in SV.
Clinical perspective
Heart failure patients with LV dyssynchrony can be treated by cardiac resynchronization therapy (CRT). Recently, intracardiac flow dynamics have been studied in heart failure patients with implanted CRT. Goliasch et al. reported that the timing of the onset of the diastolic vortex from mitral valve opening was delayed during deactivation of CRT and it was recovered instantly by reactivation [19]. Also, Pedrizzeti et al. found that the orientation of blood flow momentum was changed by active CRT in good responders [20]. In the present study, we found changes in intraventricular pressure difference in LV dyssynchrony. Because the present study was still in the experimental stage, further studies are needed to examine whether intraventricular pressure characteristics can be an additional marker of response to CRT in clinical settings.
Limitations
Dog’s heart rate was high especially during pacing, which hinders precise evaluation of IVPD during early and late filling phase. Also, this limitation may cause underestimation of true IVPD. Tanaka et al. compared IVPD obtained by RPI with two pressure sensors using a LV phantom to validate the RPI method. They reported that peak diastolic IVPD by RPI was underestimated by about 35% mainly because of insufficient frame rate of color Doppler echocardiography [9]. We compared IVPDs between RA pacing and RV pacing at the similar heart rate. Therefore, the effect of underestimation may be minimized. We used different dogs for RV pacing and RA pacing because we wanted to avoid a possible cardiac damage from a pacing protocol and the other running experimental protocol. Intraventricular pressure gradient has three-dimensional distribution. However, VFM is not applied to three-dimensional echocardiography at present. The existence of through-plane flows may contribute partly to underestimation of IVPD. To improve the accuracy of IVPD measurement using RPI, three-dimensional VFM is needed.
Conclusion
RPI based on VFM showed changes of intraventricular pressure distribution and difference in LV dyssynchrony induced by RV pacing. IVPD during isovolumic relaxation time was decreased by LV dyssynchrony. Decrease in IVPD during isovolumic relaxation time may reflect reduction of suction and decrease stroke volume.
References
Yotti R, Bermejo J, Benito Y, Antoranz JC, Desco MM, Rodríguez-Pérez D, Cortina C, Mombiela T, Barrio A, Elízaga J, Fernández-Avilés F (2011) Noninvasive estimation of the rate of relaxation by the analysis of intraventricular pressure gradients. Circ Cardiovasc Imaging 4:94–104
Yotti R, Bermejo J, Antoranz JC, Rojo-Alvarez JL, Allue C, Silva J, Desco MM, Moreno M, Garcia-Fernández MA (2004) Noninvasive assessment of ejection intraventricular pressure gradients. J Am Coll Cardiol 43:1654–1662
Firstenberg MS, Smedira NG, Greenberg NL, Prior DL, McCarthy PM, Garcia MJ, Thomas JD (2001) Relationship between early diastolic intraventricular pressure gradients, an index of elastic recoil, and improvements in systolic and diastolic function. Circulation 104:I330–I335
Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD (2008) Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol 294:H505–H513
Yotti R, Bermejo J, Antoranz JC, Desco MM, Cortina C, Rojo-Alvarez JL, Allué C, Martín L, Moreno M, Serrano JA, Muñoz R, García-Fernández MA (2005) A noninvasive method for assessing impaired diastolic suction in patients with dilated cardiomyopathy. Circulation 112:2921–2929
Nogami Y, Ishizu T, Atsumi A, Yamamoto M, Nakamura A, Machino-Ohtsuka T, Kawamura R, Seo Y, Aonuma K (2014) Diastolic suction in heart failure: impact of left ventricular geometry, untwist, and flow mechanics. Life Sci 102:111–117
Ohara T, Niebel CL, Stewart KC, Charonko JJ, Pu M, Vlachos PP, Little WC (2012) Loss of adrenergic augmentation of diastolic intra-LV pressure difference in patients with diastolic dysfunction: evaluation by colour M-mode echocardiography. JACC Cardiovasc Imaging 5:861–870
Stugaard M, Koriyama H, Katsuki K, Masuda K, Asanuma T, Takeda Y, Sakata Y, Itatani K, Nakatani S (2015) Energy loss in the left ventricle obtained by vector flow mapping as a new quantitative measure of severity of aortic regurgitation: a combined experimental and clinical study. Eur Heart J Cardiovasc Imaging 16:723–730
Tanaka T, Okada T, Nishimura T, Seki Y (2017) Relative pressure imaging in left ventricle using ultrasonic vector flow mapping. Jpn J Appl Phys 56:07–26
Gürel E, Prinz C, Van Casteren L, Gao H, Willems R, Voigt JU (2016) The impact of function-flow interaction on left ventricular efficiency in patients with conduction abnormalities: a particle image velocimetry and tissue Doppler study. J Am Soc Echocardiogr 29:431–440
Omar AM, Bansal M, Sengupta PP (2016) Advances in echocardiographic imaging in heart failure with reduced and preserved ejection fraction. Circ Res 119:357–374
Itatani K, Okada T, Uejima T, Tanaka T, Ono M, Miyaji K, Takenaka K (2013) Intraventricular flow velocity vector visualization based on the continuity equation and measurements of vorticity and wall shear stress. Jpn J Appl Phys 52:07–16
Asami R, Tanaka T, Kawabata KI, Hashiba K, Okada T, Nishiyama T (2017) Accuracy and limitations of vector flow mapping: left ventricular phantom validation using stereo particle image velocimetory. J Echocardiogr 15:57–66
Firstenberg MS, Greenberg NL, Garcia MJ, Thomas JD (2008) Relationship between ventricular contractility and early diastolic intraventricular pressure gradients: a diastolic link to systolic function. J Am Soc Echocardiogr 21:501–506
Yanada A, Ohte N, Narita H, Akita S, Miyabe H, Takada N, Goto T, Mukai S, Hayano J, Kimura G (2003) The role of apically directed intraventricular isovolumic relaxation flow in speeding early diastolic left ventricular filling. J Am Soc Echocardiogr 16:1226–1230
Lin MS, Lin JL, Liu YB, Wu CC, Lin LC, Chen MF (2006) Immediate impairment of left ventricular mechanical performance and force-frequency relation by rate-responsive dual-chamber, but not atrial pacing: Implications from intraventricular isovolumic relaxation flow. Int J Cardiol 109:367–374
Nakatani S, Beppu S, Nagata S, Ishikura F, Tamai J, Yamagishi M, Ohmori F, Kimura K, Takamiya M, Miyatake K (1994) Diastolic suction in the human ventricle: observation during balloon mitral valvuloplasty with a single balloon. Am Heart J 127:143–147
Udelson JE, Bacharach SL, Cannon RO 3rd, Bonow RO (1990) Minimum left ventricular pressure during beta-adrenergic stimulation in human subjects. evidence for elastic recoil and diastolic “suction” in the normal heart. Circulation 82:1174–1182
Goliasch G, Goscinska-Bis K, Caracciolo G, Nakabo A, Smolka G, Pedrizzetti G, Narula J, Sengupta PP (2013) CRT improves LV filling dynamics: insights from echocardiographic particle imaging velocimetry. JACC Cardiovasc Imaging 6:704–713
Pedrizzetti G, Martiniello AR, Bianchi V, D’Onofrio A, Caso P, Tonti G (2016) Changes in electrical activation modify the orientation of left ventricular flow momentum: novel observations using echocardiographic particle image velocimetry. Eur Heart J Cardiovasc Imaging 17:203–209
Acknowledgements
We thank Mr. Takashi Okada (Hitachi) and Mr. Tomohiko Tanaka (Hitachi) for technical advice and development of vector flow mapping.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Nakatani has received a research grant from Hitachi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Minami, S., Masuda, K., Stugaard, M. et al. Noninvasive assessment of intraventricular pressure difference in left ventricular dyssynchrony using vector flow mapping. Heart Vessels 36, 92–98 (2021). https://doi.org/10.1007/s00380-020-01664-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-020-01664-3