Abstract
It is unclear whether the electrocardiogram amplitude in the inferior leads (Amp-I) can always predict the height of the origin of right ventricular outflow tract arrhythmias (RVOT-VAs). We analyzed patients who received catheter ablation of multiple RVOT-VAs in the same session in our hospital from 2011 to 2016. Two distinguished RVOT-VAs, those with anatomically higher origins (HOs) and lower origins (LOs), were identified and compared to measure the longitudinal distance. Amp-I was uniquely determined for each OTVA as the highest amplitude in leads II, III, and aVF and compared between the HO-VAs and LO-VAs. In total, out of 187 patients who underwent catheter ablation of RVOT-VAs, 9 (4.8%) had multiple right OTVAs successfully treated. Four cases (Group A) had HO-VAs (10.8 ± 5.3 mm from an LO) with a lower Amp-I (1.28 ± 0.46 mV) than the LO-VAs (1.81 ± 0.59 mV), whereas the other 4 patients (Group B) had HO-VAs with a higher Amp-I (1.91 ± 0.23 mV) than the LO-VAs (1.26 ± 0.35 mV). In Group A, all HO-VAs originated from the lateral free wall and had notched R waves in the inferior leads, whereas all LOs with higher Amp-Is were located on the septum. In one patient, the HO and LO were at almost the same height, while a VA from a lateral origin had lower notched R waves in the inferior leads. A divided excitation from high lateral origins may result in not only QRS notching, but also a reduction in the QRS amplitude. In patients harboring multiple RVOT-VAs, VAs arising from the high lateral free wall could have lower Amp-Is than VAs from low septal origins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The right ventricular outflow tract (RVOT) is the most common origin of idiopathic ventricular arrhythmias (VAs). Typical morphologies on the surface 12-lead electrocardiogram (ECG) of RVOT-VAs are characterized by monophasic positive R waves in the inferior leads and a late transitional zone in the precordial leads. Because a high prevalence of VAs may lead to an impaired left ventricular performance, as well as significant symptoms [1, 2], interventional treatment should be considered. Radiofrequency catheter ablation (RFCA) is a standard curative therapy for eliminating RVOT-VAs owing to a high efficacy, low incidence of complications, and reversal effect of VA-induced cardiomyopathy [3, 4]. Although the success rate of intervention for RVOT-VAs is considered to be high, a preprocedural prediction of the origin using the surface ECG is still important for reducing the radiation exposure and procedure time [5,6,7]. In general, a higher amplitude of the tallest R wave in the inferior leads (Amp-I) on the twelve-lead electrocardiogram (ECG) is considered to be related to an anatomically higher origin of the VA. On the other hand, Dixit et al. previously reported in a pacing study that the Amp-I of the paced QRS on the free wall is lower than that on the septum in the same horizontal plane [8], but its clinical implication remains to be elucidated. In the present study, we investigated multiple RVOT-VA cases to assess the relationship between the Amp-I and anatomical location of the origin.
Methods
Study population
We investigated 187 consecutive patients without organic heart disease who underwent their first RFCA for VAs originating from the outflow tract in our facility. In those subjects, a total of 9 patients (age 45.8 ± 16.7 years, 4 females) harboring two or more distinguished RVOT-VAs were finally enrolled for further study. As we regarded the successful point as the origin of VAs, RVOT-VAs were defined as VAs successfully eliminated by ablation exclusively performed in RVOTs. Any VAs eliminated by ablation in the left ventricular out flow tracts or the coronary veins were excluded. Preprocedural 12-lead ECGs and transthoracic echocardiography were performed in all cases. Holter monitoring was performed in all cases except for in one prior to the procedure. All the patients gave written informed consent prior to the procedure. The study protocol was approved by the hospital’s institutional review board. The study complied with the Declaration of Helsinki.
Ablation procedure
All antiarrhythmic drugs were discontinued for at least five half-lives prior to the procedure. The surface electrocardiogram and bipolar intracardiac electrograms were continuously monitored and stored on a computer-based digital recording system (LabSystem PRO, Bard Electrophysiology, Lowell, MA, USA). The bipolar electrograms were filtered from 30 to 500 Hz. A 5 Fr quadripolar mapping catheter was inserted through the right femoral vein into the right ventricular apex for pacing and recording. All procedures were performed with conventional fluoroscopic guidance. Three-dimensional mapping system (CARTO XP™/CARTO3™, Biosense-Webster, Diamond Bar, CA, USA) was also used in some cases. Fluoroscopic images were always obtained in a 35° right anterior oblique view (RAO) and 45° left anterior oblique view (LAO).
A 5000 IU infusion of heparin was administered immediately following the vascular access. An ablation catheter was delivered into the RVOT via a femoral access. The earliest activation site was determined by contact mapping of the VAs and pace-mapping maneuvers were also performed. As for the non-irrigated 4 mm tip catheters, radiofrequency energy was applied with a temperature limit set at 55 °C and output limit of 50 W. As for the open-irrigated catheters, the maximum output was set at 35 W. Elimination of the target VAs was confirmed under an infusion of isoproterenol and adenosine triphosphate unless contraindicated. All successful sites were recorded by fluoroscopic images.
Comparison of multiple RVOT-VAs
To evaluate the relative change in the electroanatomic characteristics of the multiple RVOT-VAs in each patient, we selected two distinguished VAs for the analyses. If there were three or more VAs in a single patient, the anatomically highest and the lowest origins were selected for further analyses as described below.
Anatomic location of the origins of the RVOT-VAs
We quantified the spatial distribution of the two VAs in each patient. Anatomically higher origins (HOs) and lower origins (LOs) were identified in every patient as shown in Fig. 1. The longitudinal distance between the HO and LO were measured on the fluoroscopic images by LAO view by measuring longitudinal distance from a specific reference point (e.g., edge of vertebrae) to each ablation site. The horizontal location of the origin was also examined by an LAO fluoroscopic view to determine whether it was located on the septum or free wall.
Evaluation of the ECG findings
The characteristics of the twelve-lead ECGs of all VAs were evaluated focusing on the Amp-I, notch in the QRS in the inferior leads, morphology in lead-I, and transitional zone in the precordial leads. The Amp-I was uniquely determined for each VA according to the amplitude in lead-II. We evaluated the Amp-I of two separately distinguished VAs in each patient. To quantify the difference in the Amp-I of the two VAs, the ΔAmp-I was defined as the value obtained by subtracting Amp-I of the LO-VA from that of the HO-VA. The activation axis of the native QRS during sinus rhythm was also calculated.
Statistics
All statistical analyses were performed using R version 3.2.2 software [R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/]. The continuous variables were reported as the means ± standard deviations and were compared using a Student’s t test. The differences between the proportions were compared using Fisher’s exact tests. All p values were two sided, and statistical significance was established at a P < 0.05.
Results
Patient characteristics and procedural results
The patient characteristics are shown in Table 1. In the total 9 patients, three had underlying disease, whereas no patients had any organic heart disease. As for echocardiographic measurements, the mean left ventricular ejection fraction was 60.0 ± 5.7% and the mean left ventricular diastolic diameter index was 29.3 ± 1.7 mm/m2. The mean preprocedural prevalence of VAs was 20780 ± 14401/day, and ventricular tachycardia was observed in 6 patients (67%). We identified 2 separately distinguished RVOT-VAs arising from the LO and HO in 7 patients, and 2 patients exhibited 3 RVOT-VAs. All target VAs were successfully mapped and eliminated by RFCA, while no procedure-related complications were observed.
Evaluation of the ECG findings and location of the origins
A summary of the ECG findings and anatomic location of each VA are shown in Table 2. All Amp-Is were obtained from lead-II. The Amp-I of the LO-VAs and HO-VAs were 1.52 ± 0.52 and 1.55 ± 0.47 mV, respectively. The location of the origins, Amp-I, and the presence of a notched QRS in the inferior leads were individually described for the LO-VAs and HO-VAs. In Cases 1–4 (Group A), the HO-VAs had a lower Amp-I (1.28 ± 0.46 mV) and the LO-VAs had a higher Amp-I (1.81 ± 0.59 mV) irrespective of whether or not the HO was located non-negligibly higher than the LO (longitudinal distance: 10.8 ± 5.3 mm). Thus, the ΔAmp-I had a negative value in those four cases (− 0.53 ± 0.15 mV) and correlated inversely with the anatomic height of the origins. As for those cases, all LOs were located on the septal wall, whereas all the HOs were located on the lateral free wall. It was also notable that the HO-VAs of all 4 cases in Group A had a notch on the R wave. In the four Case 5–8 patients (Group B), the HO-VAs exhibited a higher Amp-I (1.91 ± 0.23 mV) than the LO-VAs (1.26 ± 0.35 mV), so that the ΔAmp-I had a positive value (0.66 ± 0.31 mV) and was positively correlated with the anatomic height of the origins. In Group B, notched R waves in the HO-VAs were observed only in Case 6. In Case 9, a VA arising from the lateral free wall had a notched QRS with a lower Amp-I than that from the septum (1.17 vs. 1.37 mV, ΔAmp-I = − 0.20 mV), while the heights of the LO and HO were almost the same. Because the ventricular activation vector could be affected by the position of the heart, we compared native QRS axis between Group A and Group B. However, there was no significant difference (+ 53.5° ± 21.3° vs. + 74.8° ± 10.3°, P = 0.265).
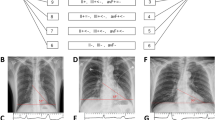
Figure 2 shows the 12-lead ECGs of all the cases in Group A, who exhibited an inverse correlation between the anatomic height and ΔAmp-I. In Case 1, a VA with a higher Amp-I (1.70 mV) originated from the low septum, whereas another one with a lower Amp-I (1.07 mV) and notched QRS was successfully ablated on the high lateral free wall (Fig. 3a). The longitudinal distance (LD) between the origins was substantially distant and was 13 mm. As the former VA was mapped and ablated first, the lower lateral region was consequently mapped for the latter VA and took a significant time to reach the actual high origin. On the other hand, in Case 6, a VA with a higher Amp-I (1.88 mV) and another VA with a lower Amp-I (1.43 mV) arose from the high septum and low septum (LD = 4 mm), respectively (Fig. 3b).
ECG from four cases in Group A who had VAs arising from higher origins with a lower amplitude in the inferior leads than that of those with lower origins. It is notable that the VAs from higher origins had a notched R wave in all four cases. HO anatomically higher origin, LO anatomically lower origin, VA ventricular arrhythmia
Representative case of an inverse and positive correlation between the anatomic height and amplitude in the inferior leads. a The ECG and fluoroscopic view of each ventricular arrhythmia in Case 1. The right panel shows a VA arising from a high lateral origin exhibiting a lower amplitude in the inferior leads than that of the lower septal origin shown in the left panel. b The ECG and fluoroscopic view of each ventricular arrhythmia in Case 6. Both VAs arose from the septal wall. A higher origin generated greater amplitude in the inferior leads as shown in the left panel. HO anatomically higher origin, LAO left oblique view, LO anatomically lower origin, RAO right oblique view
Discussion
To the best of our knowledge, this is the first report describing the inverse correlation of location of the anatomic origin and Amp-I in multiple RVOT-VA cases. Our study reconfirmed the same phenomenon reported in some preceding studies, not by pacing maneuvers, but in multiple VAs actually arising from RVOT. In the present study, we found that (1) 4 Group A cases exhibited an inverse correlation between the anatomic height and Amp-I, (2) the HOs were always located on the lateral free wall and the LOs on the septum in Group A, and (3) all VAs originating from the lateral free wall had a notched QRS wave in the Group A cases.
Role of the Amp-I in predicting the anatomic height of the OT-VAs
In general, a higher origin of a VA provokes a dominantly downward and less upward vector of the ventricular myocardial excitation. This results in an increased amplitude of the R wave of the VA without a negative component in the inferior leads of the 12-lead ECG. Evaluating the amplitude of the R wave in the inferior leads is important for predicting the height of the origin prior to the ablation procedure of the VAs. In particular, in multiple-VA cases, a relative alteration in the 12-lead ECG from one to another VA is important for predicting the spatial relationship of the origins. There have already been some reports of an alteration in the ECG characteristics in patients harbouring multiple idiopathic OT-VAs, including left-sided origins [9, 10]. Kaseno et al. reported that VAs arising from the left sinus of Valsalva (LSV) were associated with a higher Amp-I than endocardial VAs from the outflow tract, because the LSV is located at a higher position than the endocardial sites [9].
Septal origins vs. lateral origins
In the preceding context, an increased Amp-I tended to be construed as a higher anatomic origin. On the other hand, Tada et al. reported the Amp-I of lateral origin RVOT-VAs was significantly smaller than that of septal origins in a series of 110 patients [11]. However, they compared VAs of different patients, not in same patients. Thus, those results may potentially have been affected by patient-specific factors. Yet, regarding the pacing study, Dixit et al. reported a significant reduction in the Amp-I of the paced QRS on the free wall as compared to that on the septum if they were within a single horizontal plane [8]. In terms of the LVOT, Hachiya et al. reported that pacing from the distal great cardiac vein (d-GCV) created lower R waves in the inferior leads than that in the anterior interventricular vein (AIV), even if the AIV was located lower than the d-GCV [12]. Those reports commonly suggested that VAs arising from a structure off the interventricular septum would have a significantly lower Amp-I than those from septal origins. In Case 9, we confirmed that this phenomenon was practical for clinical RVOT-VAs arising from the lateral and septal walls at the same height. Moreover, it should be emphasized that this relationship was still effective even if the lateral origin was located higher than the septal origin in the same patient of Group A. This fact implies that not only the Amp-I, but also other ECG morphologies should be synthetically examined to predict the anatomic height of the origin.
In the total 9 VAs arising from the lateral wall, 7 had discernible notched R waves. Indeed, all laterally located HO-VAs had a notched QRS in Group A, because the propagation of lateral RVOT-VAs infiltrates the left ventricle from the right ventricle with a slight delay and its propagation vector leans toward a posterior direction as shown in Fig. 4, which results in a split and lower R wave in then inferior leads. Therefore, it should always be presumed that RVOT-VAs with a split low R wave may be located on the free wall at a level higher than expected. Similarly, high R waves without a notch could be associated with a septal origin lower than the operator’s imagination. Although the activation of VAs may be affected by the position of the heart, there was not a statistically significant difference of the native QRS axis between Group A and Group B. It may have been because the heart axis on Einthoven’s triangle could not reflect the difference of vector on the sagittal plane in these patients of our study.
Description of the vector of the excitation and its deflection in lead-II from the septal and free wall origins. The free wall origin is located more anterior to the septal origin in the LAO view (left panel). Then, the vector of the excitation arising from the free wall origin leans toward a more posterior direction (center panel) than that from the septal origin (right panel). FW free wall, IVS interventricular septum, LV left ventricle, RV right ventricle
Benefits to evaluate Amp-I in clinical settings
If comparing multiple RVOT-VAs in a same patient, physicians should avoid a misdirection by an idea that higher Amp-I always means an anatomically higher origin. According to the results of our study, the former concept was not true. Although three-dimensional mapping systems (3DMSs) are highly effective for mapping various arrhythmias, creating activation maps on the 3DMS is not always the best method for RVOT-VAs, especially if ectopic beats are rare during the procedure. In such a situation, pace mapping directed by the insight obtained from a detailed evaluation of the surface ECG regarding the origin would be vital to carry out a successful ablation. The results of the present study may contribute to a more efficient mapping of multiple RVOT-VAs.
Limitations
We had some limitations. First, this was a retrospective single-center study consisting of a small number of patients. Second, the determination of the axial distribution of VA origins relied on fluoroscopic images, which may not have been precise enough to detect the actual anatomy. However, it is difficult to recognize what part of the ventricle an ablation catheter really contacts, but it is possible if utilizing an intracardiac echo probe. A brief determination of the catheter location may suit the actual practice of the ablation procedure. Third, the height of the origins was determined along the longitudinal axis of the body and not that of the heart. If discussing the vector of a synthesized excitation of the ventricle, the height might have to be determined along the axis of the heart. On the other hand, it is very difficult to determine the axis of the heart by fluoroscopy or echocardiography. Because we evaluated the body surface ECG in this investigation, it may be convincing to use a system with measurements depending on the body axis.
Conclusions
In patients harboring multiple RVOT-VAs, the VAs arising from the high lateral free wall may have notched QRS as well as a lower Amp-I than the VAs with a low septal origin. These findings potentially improve mapping maneuvers during ablation procedures for RVOT-VAs.
References
Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, Hatakeyama Y, Izumi T (2009) Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 95:1230–1237
Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, Armstrong W, Good E, Chugh A, Jongnarangsin K, Pelosi F Jr, Crawford T, Ebinger M, Oral H, Morady F, Bogun F (2010) Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 7:865–869
Takemoto M, Yoshimura H, Ohba Y, Matsumoto Y, Yamamoto U, Mohri M, Yamamoto H, Origuchi H (2005) Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 45:1259–1265
Sekiguchi Y, Aonuma K, Yamauchi Y, Obayashi T, Niwa A, Hachiya H, Takahashi A, Nitta J, Iesaka Y, Isobe M (2005) Chronic hemodynamic effects after radiofrequency catheter ablation of frequent monomorphic ventricular premature beats. J Cardiovasc Electrophysiol 16:1057–1063
Jadonath RL, Schwartzman DS, Preminger MW, Gottlieb CD, Marchlinski FE (1995) Utility of the 12-lead electrocardiogram in localizing the origin of right ventricular outflow tract tachycardia. Am Heart J 130:1107–1113
Movsowitz C, Schwartzman D, Callans DJ, Preminger M, Zado E, Gottlieb CD, Marchlinski FE (1996) Idiopathic right ventricular outflow tract tachycardia: narrowing the anatomic location for successful ablation. Am Heart J 131:930–936
Park KM, Kim YH, Marchlinski FE (2012) Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 35:1516–1527
Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE (2003) Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol 14:1–7
Tada H, Hiratsuji T, Naito S, Kurosaki K, Ueda M, Ito S, Shinbo G, Hoshizaki H, Oshima S, Nogami A, Taniguchi K (2004) Prevalence and characteristics of idiopathic outflow tract tachycardia with QRS alteration following catheter ablation requiring additional radiofrequency ablation at a different point in the outflow tract. Pacing Clin Electrophysiol 27:1240–1249
Kaseno K, Tada H, Ito S, Tadokoro K, Hashimoto T, Miyaji K, Naito S, Oshima S, Nogami A, Taniguchi K (2007) Ablation of idiopathic ventricular tachycardia in two separate regions of the outflow tract: prevalence and electrocardiographic characteristics. Pacing Clin Electrophysiol 30(Suppl 1):S88–S93
Tada H, Ito S, Naito S, Kurosaki K, Ueda M, Shinbo G, Hoshizaki H, Oshima S, Nogami A, Taniguchi K (2004) Prevalence and electrocardiographic characteristics of idiopathic ventricular arrhythmia originating in the free wall of the right ventricular outflow tract. Circ J 68:909–914
Hachiya H, Hirao K, Nakamura H, Taniguchi H, Miyazaki S, Komatsu Y, Kusa S, Takagi T, Iwasawa J, Ichihara N, Kuroi A, Hayashi T, Tanaka Y, Iesaka Y (2015) Electrocardiographic characteristics differentiating epicardial outflow tract-ventricular arrhythmias originating from the anterior interventricular vein and distal great cardiac vein. Circ J 79:2335–2344
Acknowledgements
We would like to thank Mr. John Martin for his help in the preparation of the manuscript.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kajiyama, T., Hachiya, H., Kusa, S. et al. Electrocardiographic morphology of multiple ventricular arrhythmias originating from the right ventricular outflow tract: inverse correlation of the amplitude in the inferior leads and anatomic height of the origin. Heart Vessels 34, 324–330 (2019). https://doi.org/10.1007/s00380-018-1235-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1235-0